Brii Biosciences Provides Corporate Updates and Reports 2023 Interim Results
- Near-term revenue opportunities with PreHevbri® and strong balance sheet supporting operations through 2026
- Positive data on BRII-835 + PEG-IFN-α combination for chronic HBV treatment
- Phase 2 study to investigate BRII-835/PEG-IFN-α combination for HBV functional cure
- Acquisition of BRII-693 for combating drug-resistant bacterial infections
- Phase 2 study of BRII-296 in postpartum depression to start in Q3 2023
- None.
Insights
Analyzing...
First patient dosed in a PEG-IFN-α controlled BRII-835 + PEG-IFN-α combination Phase 2 study for HBV functional cure
First of several studies to investigate the potential of BRII-179 in enriching patients with strong intrinsic anti-HBsAg responses for curative treatments to start before the end of 2023
Regulatory submissions and preparation for launches of PreHevbri® in APAC countries and regions are underway
Near-term revenue opportunities with PreHevbri® and strong balance sheet supporting operations through 2026
Company to host earnings call on August 22 at 8:00 PM ET / August 23 at 8:00 AM HKT and an HBV R&D Day on August 24 from 1:00-2:30 PM HKT
Infectious Disease Therapeutic Area
Recently, Brii Bio executed two strategic transactions with VBI Vaccines Inc. (Nasdaq: VBIV), gaining exclusive global rights to BRII-179 and introducing PreHevbri®, a clinically differentiated prophylactic hepatitis B vaccine, to APAC countries and regions.
The addition of PreHevbri® complements Brii Bio's existing functional cure portfolio, further advancing solutions to reduce the transmission of HBV across
In June, Brii Bio's strategic partner, Vir Biotechnology, Inc. (Nasdaq: VIR), shared data from Part A of its Phase 2 MARCH trial of VIR-2218 (BRII-835) and VIR-3434 (BRII-877) at the 2023 European Association for the Study of the Liver (EASL) Congress, demonstrating significant declines in HBsAg levels and
Additionally, compelling data highlights the potential of BRII-835 (VIR-2218)/PEG-IFN-α combination as a best-in-class functional curative treatment for chronic HBV infections. Findings show that robust anti-HBs antibody responses at the end of treatment were associated with sustained HBsAg loss 24 weeks post-treatment, pointing to the important role of patients' humoral immunity in achieving sustained immune control of HBV infections.
Building upon this critical insight, Brii Bio has initiated a randomized and active-controlled BRII-835 + PEG-IFN-α Phase 2 study, following regulatory approvals from multiple regulatory authorities in APAC including the NMPA in mainland
In a separate transaction with Qpex, Brii Bio acquired exclusive global rights to BRII-693 (also previously known as QPX9003), a potentially best-in-class synthetic lipopeptide IV antibiotic for combating difficult-to-treat multi-drug- and extremely-drug-resistant (MDR/XDR) gram-negative bacterial infections (especially carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa), reinforcing the Company's commitment to addressing antibiotic resistance challenges while strengthening financial support.
Central Nervous System Disease Therapeutic Area
Following an agreement with
Additionally, the Company continues to advance a second long-acting injectable, BRII-297, in a first-in-human Phase 1 study, expanding the potentially groundbreaking treatment paradigm for various anxiety and depressive disorders or indications.
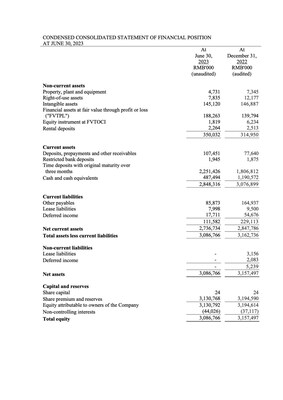
1H 2023 Financial Results
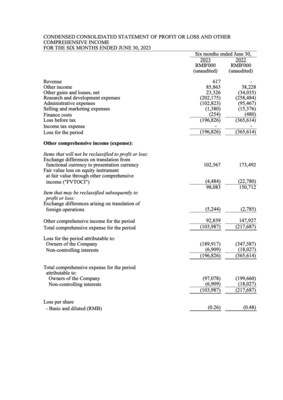
- Other Income was
RMB85.9 million for 1H 2023, representing an increase ofRMB47.7 million , or124.9% , compared withRMB38.2 million for 1H 2022. The increase was mainly due to the increased bank interest income ofRMB36.1 million attributable to the additional placement of time deposits with original maturity over three months and the increased income recognized from PRC government grants ofRMB11.6 million . - Research and development expenses were
RMB202.2 million for 1H 2023, representing a decrease ofRMB56.3 million , or21.8% , compared withRMB258.5 million for 1H 2022. The decrease was primarily due to the reduced third-party contracting fees from COVID-19 programs after the Company decided to terminate these programs. - Administrative expenses were
RMB102.8 million for 1H 2023, representing an increase ofRMB7.3 million , or7.6% , compared withRMB95.5 million for 1H 2022. The increase was primarily attributable to the increase in employee headcounts and computer software fees. - Total comprehensive expense for 1H 2023 was
RMB104.0 million , representing a decrease ofRMB113.7 million , or52.2% , compared withRMB217.7 million for 1H 2022. The decrease was primarily due to the increase in other income and decrease in the research and development expenses.
Conference Call Information
A live conference call will be hosted on August 23, 2023, at 8:00 AM Hong Kong time (August 22, 2023, at 8:00 PM
About Brii Bio
Brii Biosciences Limited ("Brii Bio", stock code: 2137.HK) is a commercial stage biotechnology company developing therapies to address major public health challenges where patients experience high unmet medical needs, limited choice and significant social stigmas. With a focus on infectious and central nervous system diseases, the Company is advancing a broad pipeline of unique therapeutic candidates with lead programs against hepatitis B viral infection (HBV), postpartum depression (PPD), and major depressive disorder (MDD). The Company is led by a visionary and experienced leadership team and has operations in key biotech hubs, including
Forward Looking Statement
The information communicated in this press release contains certain statements that are or may be forward looking. These statements typically contain words such as "will," "expects," "believes," "plans" and "anticipates," and words of similar import. By their nature, forward looking statements involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. There may be additional material risks that are currently not considered to be material or of which the Company are unaware. These forward-looking statements are not a guarantee of future performance. Against the background of these uncertainties, readers should not rely on these forward-looking statements. The Company assumes no responsibility to update forward-looking statements or to adapt them to future events or developments.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/brii-biosciences-provides-corporate-updates-and-reports-2023-interim-results-301906916.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/brii-biosciences-provides-corporate-updates-and-reports-2023-interim-results-301906916.html
SOURCE Brii Biosciences Limited