Benitec Biopharma Releases Third Quarter 2024 Financial Results and Provides Operational Update
Benitec Biopharma reported positive interim clinical trial data for the first Oculopharyngeal Muscular Dystrophy (OPMD) subject dosed with BB-301, with improvements noted in swallowing assessments. The company closed an oversubscribed $40.0 million private financing round extending cash runway through 2025. Operational updates include enrollment of 23 subjects in the OPMD Natural History Study and milestones related to BB-301 development.
- None.
- None.
Insights
-Positive Interim Clinical Trial Data for the First Oculopharyngeal Muscular Dystrophy (OPMD) Subject Dosed with BB-301 in the Phase 1b/2a Clinical Treatment Study Reported in April-
-The Second OPMD Subject was Safely Dosed with BB-301 in February-
-Additional Interim Clinical Safety Data and Clinical Efficacy Data are Expected for Several Study Subjects in the Second Half of 2024-
-23 OPMD Subjects have Enrolled into the OPMD Natural History Study with Multiple Subjects Eligible for Entry into the BB-301 Phase 1b/2a Clinical Treatment Study-
-Closed an Oversubscribed Private Placement Financing of
HAYWARD, Calif., May 13, 2024 (GLOBE NEWSWIRE) -- Benitec Biopharma Inc. (NASDAQ: BNTC) (“Benitec” or “Company”), a clinical-stage, gene therapy-focused, biotechnology company developing novel genetic medicines based on its proprietary “Silence and Replace” DNA-directed RNA interference (“ddRNAi”) platform, today announced financial results for its third fiscal quarter ended March 31, 2024. The Company has filed its quarterly report on Form 10-Q with the U.S. Securities and Exchange Commission.
“The interim clinical trial results for the first subject enrolled onto the BB-301 Phase 1b/2a Clinical Treatment Study demonstrated consistent, clinically meaningful improvements in each of the central study endpoints, with significant improvements noted across the radiographic assessments of swallowing and corresponding improvements observed for the key subject-reported outcome measure,” said Jerel A. Banks, M.D., Ph.D., Executive Chairman and Chief Executive Officer of Benitec. "We look forward to presenting the results of longer-term follow-up for the first study subject, along with the accruing data for additional study subjects, later this year”. “Following the recent financing, Benitec is well-positioned to advance the BB-301 clinical development program through the end of 2025.”
Operational Updates
On April 18th the Company announced an oversubscribed
The financing was led by Suvretta Capital Management, LLC with participation from new and existing investors including Adage Capital Partners L.P., Nantahala Capital, multiple healthcare-focused funds, and a leading mutual fund. Gross proceeds from the PIPE financing totaled approximately
In connection with the PIPE financing, the Company has agreed with long-term investor Suvretta Capital Management to consider Kishen Mehta, a portfolio manager at Suvretta Capital Management, for appointment to the Company’s board of directors.
The key milestones related to the development of BB-301 for the treatment of OPMD-related Dysphagia, along with other corporate updates, are outlined below:
Interim BB-301 Phase 1b/2a Clinical Treatment Study Results for Subject 1:
- During the OPMD Natural History (NH) Study, which represents the pre-dose observational period for each subject, Subject 1 experienced progressive worsening of dysphagia as demonstrated by the results of the videofluoroscopic swallowing studies (VFSS), the cold water timed drinking test, and the key subject-reported outcome measure (the Sydney Swallow Questionnaire). VFSS represents the gold standard analytical method for the quantitative assessment of dysphagia (swallowing difficulty) in the clinical setting.
- At the 90-day post-dose assessment following the administration of BB-301, Subject 1 demonstrated improvements in key videofluoroscopic assessments which correlated with the observation of similar improvement in the key subject-reported outcome measure as compared to the average values for the respective assessments completed during the pre-dose observational period (as summarized in Figure 1). Notably, the results of many assessments completed at the 90-day timepoint demonstrated improvements over the initial measurements assessed at the subject’s first visit for the natural history observational study which occurred more than 12 months prior to the 90-day post-dose assessment.
- The most significant VFSS improvements at Day 90 were observed for swallowing tasks centered on the evaluation of pharyngeal constrictor muscle function and swallowing efficiency in the context of the consumption of thin liquids (e.g., water), solid foods, and thick non-solid foods (e.g., yogurt or pudding) (Figure 1). The VFSS improvements correlated with an improvement in the key subject-reported outcome measure (the Sydney Swallow Questionnaire), indicating an improvement in swallowing function as reported by Subject 1 (Figure 1).
Figure 1: Improvement in All Outcomes at 90-Days Post-BB-301 Injection*
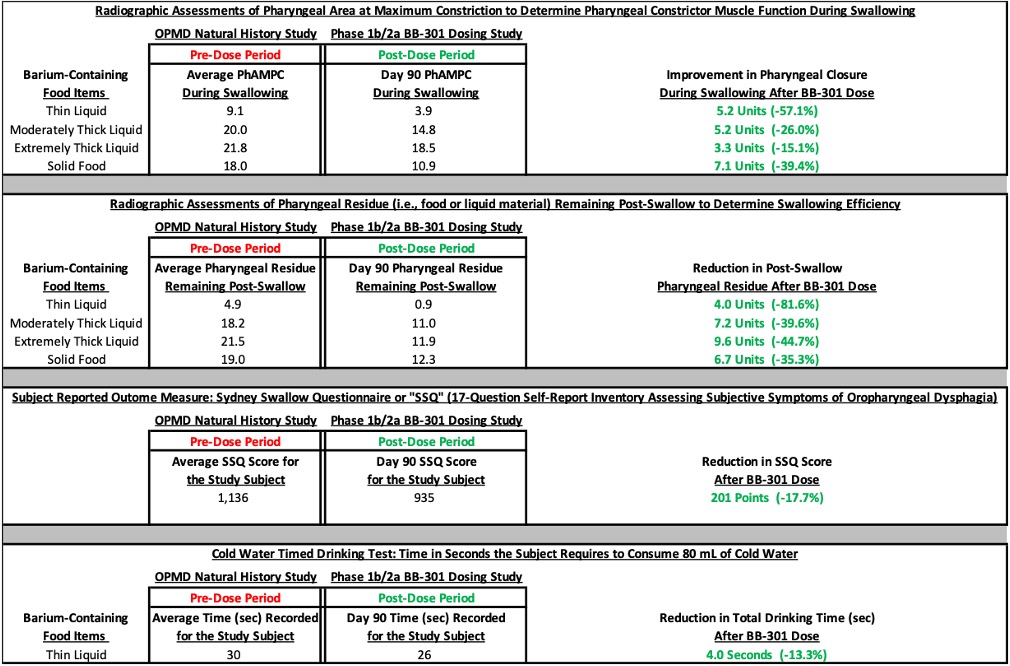
*Company data on file
- Regarding the BB-301 safety profile observed to date, no Serious Adverse Events (SAEs) have been observed for the two subjects that have received BB-301. Transient Grade 2 Gastroesophageal Reflux Disease or “GERD” (i.e., “acid reflux” or “heartburn”) was observed for the two subjects that received BB-301. For both study subjects, the GERD resolved following the use of common prescription medications approved for the treatment of GERD.
Corporate Updates:
- Twenty-three (23) subjects have been enrolled into the OPMD NH Study, and the BB-301 Phase 1b/2a Clinical Treatment Study is designed to treat 21-to-30 subjects. Two (2) subjects have rolled over from the OPMD NH Study into the BB-301 Phase 1b/2a Clinical Treatment Study, and a third OPMD subject is expected to enter the BB-301 Phase 1b/2a Clinical Treatment Study in June 2024.
- Additional interim safety data and efficacy data are expected to be reported from the BB-301 Phase 1b/2a Clinical Treatment Study in 2H 2024.
Financial Highlights
Third Fiscal Quarter 2024 Financial Results
Total Revenues for the quarter ended March 31, 2024, were
Total Expenses for the quarter ended March 31, 2024, were
The loss from operations for the quarter ended March 31, 2024, was
| BENITEC BIOPHARMA INC. Consolidated Balance Sheets (in thousands, except par value and share amounts) | |||||||
| March 31, | June 30, | ||||||
| 2024 | 2023 | ||||||
| (Unaudited) | |||||||
| Assets | |||||||
| Current assets: | |||||||
| Cash at bank | $ | 14,143 | $ | 2,477 | |||
| Restricted cash | 13 | 13 | |||||
| Trade and other receivables | 53 | 55 | |||||
| Prepaid and other assets | 157 | 1,184 | |||||
| Total current assets | 14,366 | 3,729 | |||||
| Property and equipment, net | 204 | 87 | |||||
| Deposits | 25 | 25 | |||||
| Prepaid and other assets | 69 | 97 | |||||
| Right-of-use assets | 335 | 526 | |||||
| Total assets | $ | 14,999 | $ | 4,464 | |||
| Liabilities and stockholders’ equity | |||||||
| Current liabilities: | |||||||
| Trade and other payables | $ | 2,628 | $ | 3,231 | |||
| Accrued employee benefits | 517 | 472 | |||||
| Lease liabilities, current portion | 292 | 275 | |||||
| Total current liabilities | 3,437 | 3,978 | |||||
| Lease liabilities, less current portion | 62 | 284 | |||||
| Total liabilities | 3,499 | 4,262 | |||||
| Commitments and contingencies (Note 11) | |||||||
| Stockholders’ equity: | |||||||
| Common stock, | — | — | |||||
| Additional paid-in capital | 197,255 | 168,921 | |||||
| Accumulated deficit | (184,920 | ) | (167,889 | ) | |||
| Accumulated other comprehensive loss | (835 | ) | (830 | ) | |||
| Total stockholders’ equity | 11,500 | 202 | |||||
| Total liabilities and stockholders’ equity | $ | 14,999 | $ | 4,464 | |||
The accompanying notes are an integral part of these consolidated financial statements.
| BENITEC BIOPHARMA INC. Consolidated Statements of Operations and Comprehensive Loss (Unaudited) (in thousands, except share and per share amounts) | |||||||||||||||
| Three Months Ended | Nine Months Ended | ||||||||||||||
| March 31, | March 31, | ||||||||||||||
| 2024 | 2023 | 2024 | 2023 | ||||||||||||
| Revenue: | |||||||||||||||
| Licensing revenues from customers | $ | — | $ | 54 | $ | — | $ | 68 | |||||||
| Total revenues | — | 54 | — | 68 | |||||||||||
| Operating expenses: | |||||||||||||||
| Royalties and license fees | (3 | ) | — | (108 | ) | — | |||||||||
| Research and development | 2,566 | 3,167 | 12,097 | 9,588 | |||||||||||
| General and administrative | 1,578 | 1,228 | 4,953 | 5,011 | |||||||||||
| Total operating expenses | 4,141 | 4,395 | 16,942 | 14,599 | |||||||||||
| Loss from operations | (4,141 | ) | (4,341 | ) | (16,942 | ) | (14,531 | ) | |||||||
| Other income (loss): | |||||||||||||||
| Foreign currency transaction loss | (118 | ) | (45 | ) | (22 | ) | (391 | ) | |||||||
| Interest expense, net | (4 | ) | (7 | ) | (16 | ) | (25 | ) | |||||||
| Other income (expense), net | (16 | ) | — | (50 | ) | 50 | |||||||||
| Unrealized loss on investment | — | (4 | ) | (1 | ) | (4 | ) | ||||||||
| Total other income (loss), net | (138 | ) | (56 | ) | (89 | ) | (370 | ) | |||||||
| Net loss | $ | (4,279 | ) | $ | (4,397 | ) | $ | (17,031 | ) | $ | (14,901 | ) | |||
| Other comprehensive income (loss): | |||||||||||||||
| Unrealized foreign currency translation gain (loss) | 117 | 45 | (5 | ) | 392 | ||||||||||
| Total other comprehensive income (loss) | 117 | 45 | (5 | ) | 392 | ||||||||||
| Total comprehensive loss | $ | (4,162 | ) | $ | (4,352 | ) | $ | (17,036 | ) | $ | (14,509 | ) | |||
| Net loss | $ | (4,279 | ) | $ | (4,397 | ) | $ | (17,031 | ) | $ | (14,901 | ) | |||
| Net loss per share: | |||||||||||||||
| Basic and diluted | $ | (1.64 | ) | $ | (2.67 | ) | $ | (6.95 | ) | $ | (11.47 | ) | |||
| Weighted average number of shares outstanding: basic and diluted | 2,616,288 | 1,645,951 | 2,449,295 | 1,299,423 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
About BB-301
BB-301 is a novel, modified AAV9 capsid expressing a unique, single bifunctional construct promoting co-expression of both codon-optimized Poly-A Binding Protein Nuclear-1 (PABPN1) and two small inhibitory RNAs (siRNAs) against mutant PABPN1. The two siRNAs are modeled into microRNA backbones to silence expression of faulty mutant PABPN1, while allowing expression of the codon-optimized PABPN1 to replace the mutant with a functional version of the protein. We believe the silence and replace mechanism of BB-301 is uniquely positioned for the treatment of OPMD by halting mutant expression while providing a functional replacement protein.
About Benitec Biopharma, Inc.
Benitec Biopharma Inc. (“Benitec” or the “Company”) is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary “Silence and Replace” DNA-directed RNA interference platform combines RNA interference, or RNAi, with gene therapy to create medicines that simultaneously facilitate sustained silencing of disease-causing genes and concomitant delivery of wildtype replacement genes following a single administration of the therapeutic construct. The Company is developing Silence and Replace-based therapeutics for chronic and life-threatening human conditions including Oculopharyngeal Muscular Dystrophy (OPMD). A comprehensive overview of the Company can be found on Benitec’s website at www.benitec.com.
Forward Looking Statements
Except for the historical information set forth herein, the matters set forth in this press release include forward-looking statements, including statements regarding Benitec’s plans to develop and commercialize its product candidates, the timing of the completion of pre-clinical and clinical trials, the timing of the availability of data from our clinical trials, the timing and sufficiency of patient enrollment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and Benitec’s product candidates, the intellectual property position, the length of time over which the Company expects its cash and cash equivalents to be sufficient to execute on its business plan, and other forward-looking statements.
These forward-looking statements are based on the Company’s current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities and other regulatory developments; the Company’s ability to protect and enforce its patents and other intellectual property rights; the Company’s dependence on its relationships with its collaboration partners and other third parties; the efficacy or safety of the Company’s products and the products of the Company’s collaboration partners; the acceptance of the Company’s products and the products of the Company’s collaboration partners in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; greater than expected expenses; expenses relating to litigation or strategic activities; the Company’s ability to satisfy its capital needs through increasing its revenue and obtaining additional financing; the impact of the COVID-19 pandemic, the disease caused by the SARS-CoV-2 virus or any similar events, which may adversely impact the Company’s business and pre-clinical and clinical trials; the impact of local, regional, and national and international economic conditions and events; and other risks detailed from time to time in the Company’s reports filed with the Securities and Exchange Commission. The Company disclaims any intent or obligation to update these forward-looking statements.
Investor Relations Contact:
Irina Koffler
LifeSci Advisors, LLC
(917) 734-7387
ikoffler@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/8bb63807-b739-42f2-a124-8caa83944152







