Four New Studies Demonstrate that Viz.ai Finds New Patients with Hypertrophic Cardiomyopathy Earlier When Embedded into the Clinical Workflow
Viz.ai announced new clinical data showing the effectiveness of its Viz HCM module, developed in partnership with Bristol Myers Squibb (NYSE:BMY), in detecting hypertrophic cardiomyopathy (HCM). The module, which received FDA De Novo approval in August 2023, is the first AI algorithm cleared for HCM detection.
Four studies presented at ACC 2025 demonstrated significant results:
- Cleveland Clinic study identified 574 HCM patients with high accuracy
- 20% of patients could have been diagnosed more than one year earlier
- In a multicenter study of 145,848 screened patients, 3% were flagged for suspected HCM, leading to 17 new HCM diagnoses
- Algorithm showed 56% sensitivity and 100% specificity in detecting HCM confirmed by cardiac MRI
Viz.ai ha annunciato nuovi dati clinici che dimostrano l'efficacia del suo modulo Viz HCM, sviluppato in collaborazione con Bristol Myers Squibb (NYSE:BMY), nella rilevazione della cardiomiopatia ipertrofica (HCM). Il modulo, che ha ricevuto l'approvazione De Novo dalla FDA nell'agosto 2023, è il primo algoritmo AI autorizzato per la rilevazione dell'HCM.
Quattro studi presentati all'ACC 2025 hanno mostrato risultati significativi:
- Lo studio della Cleveland Clinic ha identificato 574 pazienti con HCM con alta precisione
- Il 20% dei pazienti avrebbe potuto essere diagnosticato più di un anno prima
- In uno studio multicentrico di 145.848 pazienti sottoposti a screening, il 3% è stato segnalato per sospetta HCM, portando a 17 nuove diagnosi di HCM
- L'algoritmo ha mostrato una sensibilità del 56% e una specificità del 100% nella rilevazione dell'HCM confermata da MRI cardiaca
Viz.ai anunció nuevos datos clínicos que muestran la efectividad de su módulo Viz HCM, desarrollado en asociación con Bristol Myers Squibb (NYSE:BMY), para detectar la cardiomiopatía hipertrófica (HCM). El módulo, que recibió la aprobación De Novo de la FDA en agosto de 2023, es el primer algoritmo de IA autorizado para la detección de HCM.
Cuatro estudios presentados en el ACC 2025 demostraron resultados significativos:
- El estudio de la Cleveland Clinic identificó 574 pacientes con HCM con alta precisión
- El 20% de los pacientes podría haber sido diagnosticado más de un año antes
- En un estudio multicéntrico de 145,848 pacientes examinados, el 3% fue señalado por sospecha de HCM, lo que llevó a 17 nuevos diagnósticos de HCM
- El algoritmo mostró una sensibilidad del 56% y una especificidad del 100% en la detección de HCM confirmada por MRI cardíaca
Viz.ai는 Bristol Myers Squibb (NYSE:BMY)와 협력하여 개발한 Viz HCM 모듈의 효과를 보여주는 새로운 임상 데이터를 발표했습니다. 이 모듈은 2023년 8월 FDA의 De Novo 승인을 받았으며, HCM 감지를 위한 최초의 AI 알고리즘입니다.
ACC 2025에서 발표된 네 가지 연구는 중요한 결과를 보여주었습니다:
- Cleveland Clinic 연구는 높은 정확도로 574명의 HCM 환자를 식별했습니다
- 환자의 20%는 1년 이상 빨리 진단받을 수 있었습니다
- 145,848명의 선별된 환자를 대상으로 한 다기관 연구에서 3%가 의심되는 HCM으로 표시되어 17개의 새로운 HCM 진단으로 이어졌습니다
- 알고리즘은 심장 MRI로 확인된 HCM 감지에서 56%의 민감도와 100%의 특이성을 보였습니다
Viz.ai a annoncé de nouvelles données cliniques montrant l'efficacité de son module Viz HCM, développé en partenariat avec Bristol Myers Squibb (NYSE:BMY), dans la détection de la cardiomyopathie hypertrophique (HCM). Le module, qui a reçu l'approbation De Novo de la FDA en août 2023, est le premier algorithme d'IA autorisé pour la détection de l'HCM.
Quatre études présentées lors de l'ACC 2025 ont démontré des résultats significatifs :
- L'étude de la Cleveland Clinic a identifié 574 patients atteints de HCM avec une grande précision
- 20% des patients auraient pu être diagnostiqués plus d'un an plus tôt
- Dans une étude multicentrique de 145 848 patients dépistés, 3% ont été signalés pour suspicion de HCM, conduisant à 17 nouveaux diagnostics de HCM
- L'algorithme a montré une sensibilité de 56% et une spécificité de 100% dans la détection de l'HCM confirmée par une IRM cardiaque
Viz.ai hat neue klinische Daten veröffentlicht, die die Wirksamkeit seines Viz HCM-Moduls zeigen, das in Zusammenarbeit mit Bristol Myers Squibb (NYSE:BMY) entwickelt wurde, um hypertrophe Kardiomyopathie (HCM) zu erkennen. Das Modul, das im August 2023 die FDA De Novo-Zulassung erhielt, ist der erste KI-Algorithmus, der zur HCM-Erkennung freigegeben wurde.
Vier Studien, die auf dem ACC 2025 vorgestellt wurden, zeigten signifikante Ergebnisse:
- Die Cleveland Clinic-Studie identifizierte 574 HCM-Patienten mit hoher Genauigkeit
- 20% der Patienten hätten mehr als ein Jahr früher diagnostiziert werden können
- In einer multizentrischen Studie mit 145.848 gescreenten Patienten wurden 3% auf verdächtige HCM hingewiesen, was zu 17 neuen HCM-Diagnosen führte
- Der Algorithmus zeigte eine Sensitivität von 56% und eine Spezifität von 100% bei der Erkennung von HCM, die durch eine kardiale MRT bestätigt wurde
- First FDA-cleared AI algorithm for HCM detection, creating new regulatory category
- Partnership with major pharmaceutical company BMY enhances market position
- 100% specificity and positive predictive value in MRI-confirmed cases
- Demonstrated ability to detect HCM years before traditional diagnosis
- Relatively low sensitivity rate of 56% in MRI-confirmed cases
- Only 17 new HCM diagnoses from large patient pool of 145,848 screened
Insights
Bristol Myers Squibb's multi-year partnership with Viz.ai is yielding significant clinical validation for their jointly developed AI technology for hypertrophic cardiomyopathy (HCM). The four studies being presented at ACC 2025 provide compelling evidence that the Viz HCM module effectively identifies patients with this commonly underdiagnosed condition much earlier in the disease course.
What's particularly notable is that this represents the first FDA-cleared AI algorithm for HCM, creating an entirely new regulatory category for cardiovascular machine learning software. This regulatory milestone establishes BMY as an innovator in the AI-enhanced diagnostics space for cardiovascular conditions.
The clinical data demonstrates impressive potential - one study showed that 20% of patients could have been diagnosed more than a year earlier, with some potentially identified up to 10 years before their actual diagnosis. This early detection capability addresses a critical gap in HCM management, where delayed diagnosis frequently leads to suboptimal outcomes.
For BMY, this partnership strategically positions them at the intersection of pharmaceutical development and AI-enabled precision medicine. By helping identify patients earlier, this technology creates a pathway for timely intervention with appropriate therapies, potentially expanding the treatable patient population for BMY's cardiovascular portfolio.
Real-world evidence to be presented at the American College of Cardiology Scientific Session 2025 demonstrates positive impact of Viz.ai on disease detection and care coordination
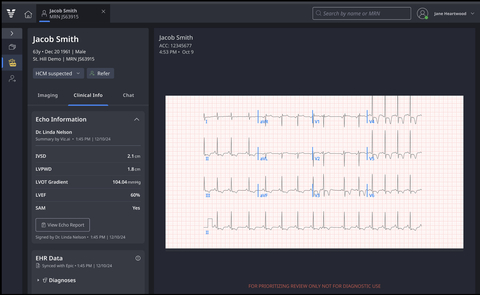
Viz HCM: Patient card displaying key clinical information, including ECG and echocardiographic findings.
“It’s exciting to see the growing real-world evidence showing how AI-enhanced ECG analysis can play a pivotal role in identifying new patients with hypertrophic cardiomyopathy,” said Milind Desai, MD, MBA, Director of the Center for Hypertrophic Cardiomyopathy at Cleveland Clinic. “By leveraging AI as a second set of eyes, we can expand the ability to diagnose more HCM patients earlier and across diverse populations, tackling a condition that’s often challenging to detect.”
Viz HCM uses artificial intelligence to analyze all 12-lead electrocardiograms (ECGs) at the point of care from across a health system to identify suspected HCM cases, notify cardiology care teams and increase the likelihood that patients get the right follow-up and diagnosis. The Viz HCM module was granted De Novo approval by the FDA in August 2023, creating a new regulatory category for cardiovascular machine learning-based notification software.
“The findings from our study highlight the potential of AI-based ECG analysis to identify hypertrophic cardiomyopathy well before a clinical diagnosis is made,” said Michael Ayers, MD, Co-Director of the HCM Center of Excellence at University of
The following clinical studies are being presented at ACC:
- “Real-World Artificial Intelligence–Based Electrocardiographic Analysis to Diagnose Hypertrophic Cardiomyopathy" evaluated the performance of Viz HCM for detecting HCM at the Cleveland Clinic. The study, published in JACC: Clinical Electrophysiology and set to be presented live at ACC 2025, demonstrated that Viz HCM achieved a high degree of accuracy in detecting HCM. The AI-ECG successfully identified 574 HCM patients, and 691 were determined to have an alternate clinically relevant diagnosis, highlighting Viz HCM’s value for more effective disease detection.
-
“A Retrospective Assessment of Delays in HCM Diagnosis and the Potential Impact of an Artificial-Intelligence-assisted Electrocardiogram Screening” used Viz HCM to predict HCM from serial 12-lead ECGs first and after which, the confirmatory diagnosis was assessed by expert clinicians at an HCM Center of Excellence. Results indicate that Viz HCM could have identified HCM patients from an ECG earlier. Among the 155 patients with AI-based ECG identifications of HCM,
20.0% could have been diagnosed more than one year prior,12.9% more than 3 years prior,9.0% more than 5 years prior, and4.5% more than 10 years prior. -
“A Multicenter, Prospective Cohort Pilot Study on the Clinical Implementation and Utilization of an AI-based ECG Tool for HCM Detection and Care Coordination” evaluated the implementation of Viz HCM into the clinical workflow to detect HCM and triage patients to the right specialist. Out of 145,848 screened patients,
3% were flagged for suspected HCM and directed to the appropriate specialist. A total of 217 patients met the study criteria and were enrolled, representing a diverse population—23% Black,9.2% Asian, and12.4% Hispanic or Latino. Out of the 217 patients, 17 new HCM patients were identified, including 8 inpatient and 9 outpatient diagnoses. The findings suggest that AI-assisted ECG screening can be successfully integrated into clinical workflows to aid in improved HCM identification and care coordination. -
“Machine-learning Algorithm for the Detection of Hypertrophic Cardiomyopathy from Standard Electrocardiogram” evaluated the performance of the Viz HCM algorithm in identifying HCM confirmed by cardiac MRI. The study found that Viz HCM identified 87 of 156 patients with HCM, rendering its sensitivity
56% , specificity100% , and positive predictive value of100% .
“At Viz.ai, we are committed to integrating AI into clinical workflows to ensure the reliable detection and timely triage of underdiagnosed conditions like HCM, ultimately enhancing care and outcomes for more patients,” said Molly Madziva Taitt, Ph.D., VP of Global Clinical Affairs at Viz.ai. “The robust clinical evidence accepted at ACC underscores the strong and consistent performance of the Viz HCM module and as a practical tool for efficiently triaging patients for clinical evaluation with the right specialist at the right time.”
To learn more about Viz.ai, visit us at ACC at booth 11055.
About Viz.ai, Inc.
Viz.ai is the pioneer in the use of AI algorithms and machine learning to increase the speed of diagnosis and care across 1,700+ hospitals and health systems in the
View source version on businesswire.com: https://www.businesswire.com/news/home/20250326744258/en/
Media Contacts
Carolyn Jones
carolyn.jones@viz.ai
Daniel Yunger
daniel.yunger@kekstcnc.com
Source: Viz.ai







