Biohaven Presents Preclinical Data Demonstrating Taldefgrobep alfa Reduces Fat and Improves Lean Mass at The Obesity Society's Annual Meeting, ObesityWeek®
- Taldefgrobep is a novel myostatin inhibitor with the potential to be an important therapy for neuromuscular disorders and metabolic diseases including obesity.
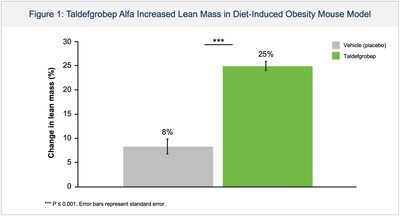
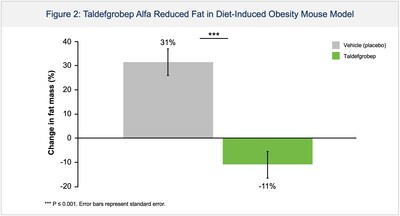
- Taldefgrobep monotherapy significantly reduced fat and increased lean muscle mass in an obese mouse model; potential body composition changes relevant to individuals living with overweight and obesity.
- Biohaven presented an analysis supporting change in waist circumference as a strong correlate for weight loss, suggesting potential alternative endpoints for obesity studies.
Poster 210: Correlation Between Reduction in Total Body Weight and Change in Waist Circumference
Poster 211: Taldefgrobep Alfa Reduces Fat and Increases Muscle in an Obese Mouse Model
The poster presentations hightlight the potential benefit of taldefgrobep in the treatment of adults living with overweight and obesity and suggest anthropometric measures of central obesity, such as waist circumference or waist-to-height ratio, may be considered as effective alternative estimations of therapeutic effect.
In a mouse model of diet-induced obesity, untreated mice exhibited an increase in fat mass of
"The disease of obesity is a public health crisis for which alternative treatment options remain necessary. With the unprecedented amount of weight loss generated by the latest anti-obesity medications, it is important that we carefully consider the quality of resulting body composition changes, including the degree of lean muscle loss. Taldefgrobep's differentiated mechanism offers the potential for a meaningful reduction in fat mass, the primary pathogenic tissue in obesity, while increasing lean mass. These body changes are unique relative to all approved anti-obesity medications which achieve reductions in total body weight based on a composite of fat mass loss and lean muscle mass loss, the latter of which may pose important adverse long-term health consequences" said Peter Ackerman, M.D., Vice President and Clinical Lead for Metabolic Disorders at Biohaven.
A Phase 2 obesity study with taldefgrobep is planned for 2024. Full posters will be available on the Posters and Presentations page at: www.biohaven.com.
Irfan Qureshi, MD, Chief Medical Officer at Biohaven, commented, "Together these posters support the development of taldefgrobep as a novel therapy for the treatment of overweight and obesity that can result in significant fat loss while increasing lean mass. This is a paradigm-shift from traditional obesity therapies that focus on overall weight loss despite significant difficult to reverse loss in muscle mass."
About Taldefgrobep alfa
Taldefgrobep alfa (BHV-2000) is a fully human recombinant protein designed to specifically designed to bind to myostatin (GDF-8) and inhibit the signaling of both myostatin and activin A, both key regulators of muscle and adipose tissue. Taldefgrobep binds myostatin. The myostatin-taldefgrobep complex then acts as an Activin 2a/2b receptor antagonist. Anti-myostatin therapy has a novel mechanism of action that may reduce fat mass, increase lean mass, and improve other metabolic parameters.
About Biohaven
Biohaven is a global clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of life-changing therapies for people with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven is advancing a pipeline of best-in-class therapies for diseases with little or no treatment options, leveraging its proven drug development capabilities and proprietary platforms, including Kv7 ion channel modulation for epilepsy and neuronal hyperexcitability; glutamate modulation for obsessive-compulsive disorder and spinocerebellar ataxia and myostatin inhibition for neuromuscular diseases. Biohaven's portfolio of early- and late-stage product candidates also includes discovery research programs focused on TRPM3 channel activation for neuropathic pain and CD-38 antibody recruiting, bispecific molecules for multiple myeloma. More information about Biohaven is available at www.biohaven.com.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "believe", "may" and "will" and similar expressions, are intended to identify forward-looking statements. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of Biohaven's management about taldefgrobep alfa as treatment for patients with neuromuscular or obesity disorders. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates are not guarantees of future performance or results and involve substantial risks and uncertainties. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven's filings with the Securities and Exchange Commission, including within the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations". The forward-looking statements are made as of the date of this new release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Biohaven Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
201-248-0741
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-presents-preclinical-data-demonstrating-taldefgrobep-alfa-reduces-fat-and-improves-lean-mass-at-the-obesity-societys-annual-meeting-obesityweek-301956902.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-presents-preclinical-data-demonstrating-taldefgrobep-alfa-reduces-fat-and-improves-lean-mass-at-the-obesity-societys-annual-meeting-obesityweek-301956902.html
SOURCE Biohaven Ltd.