Biohaven Announces Positive Data from its Exploratory Electroencephalogram (EEG) Biomarker Study of BHV-7000, Completion of Once-Daily Formulation Development, and Plan to Initiate Phase 3 Pivotal Studies
- Examination of EEG in healthy subjects administered single doses of BHV-7000 confirmed central nervous system (CNS) activity consistent with effects observed with other antiseizure medications.
- EEG results demonstrated dose-dependent and time-dependent effects of BHV-7000 on CNS target engagement in study subjects:
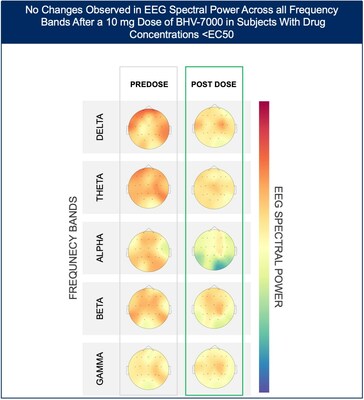
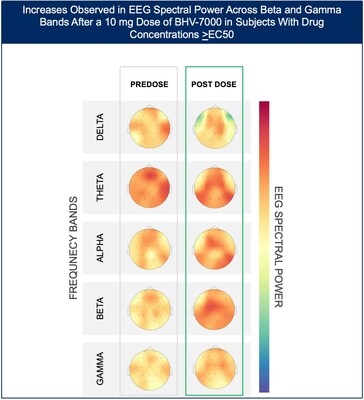
- At the lowest dose studied of 10 mg, subjects with targeted drug concentrations ≥EC50 showed a mean increase in EEG spectral power in beta and gamma bands while there were no meaningful changes in spectral power in subjects with drug concentrations <EC50.
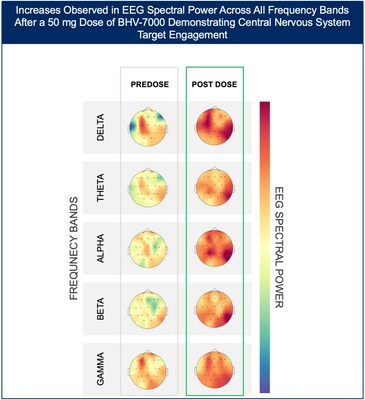
- At the highest dose studied of 50 mg, increases in EEG spectral power were observed in all frequency bands and across the entire head without distinct topographies.
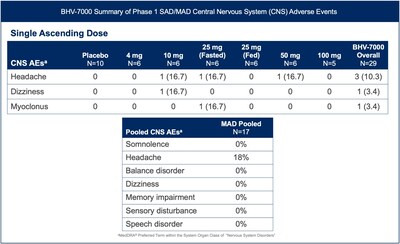
- BHV-7000 has been shown to be well tolerated in Phase 1 single ascending dose (SAD) and multiple ascending dose (MAD) studies to date, with a distinct profile from other Kv7 ion channel activators and antiseizure medications.
- Biohaven has also successfully completed the development of an extended-release formulation of BHV-7000 to allow for once-daily dosing to be studied in future clinical programs.
- With target engagement now confirmed in the biomarker EEG study, favorable safety profile demonstrated in Phase 1 studies and development of a once-daily formulation of BHV-7000, Biohaven plans to initiate its Phase 3 program in focal epilepsy before the end of 2023.
NEW HAVEN, Conn. and
Michael Bozik, M.D., President, Ion Channel Research & Development at Biohaven, commented "The EEG biomarker data show effects of BHV-7000 on CNS activity and are incredibly exciting because they further support the paradigm-changing potential of our highly selective Kv7.2/7.3 activator. Together with the preclinical data and the favorable safety and tolerability profile seen in the Phase 1 SAD/MAD studies, the EEG results highlight BHV-7000's potential to deliver robust antiseizure efficacy, without the burdensome CNS adverse effects observed with antiseizure medicines. We are extremely pleased that the BHV-7000 development program continues to advance towards our planned Phase 3 start in the fourth quarter of 2023."
The Phase 1 EEG study was designed to evaluate qualitative changes from baseline in EEG spectral power after administration of single doses of BHV-7000 (10, 25, or 50 mg) to healthy volunteers. EEG spectral power is a measure derived from quantitative analysis of EEG signals that assesses the amount of rhythmic activity in different frequency bands, including delta [1-3.5 Hz], theta [3.5-7.5 Hz], alpha [7.5-13 Hz], beta [13-30 Hz], and gamma [30-100 Hz]. Changes in spectral power have been used to evaluate the risk, onset and progression of seizures, assess cognitive and behavioral impairments, and characterize the effects of ASMs; and, they may also have utility in refining dose selection in clinical trials of ASMs. Spectral analysis was performed by Epilog (Ghent,
The Phase 1 EEG study showed dose-dependent and time-dependent increases in brain spectral power in healthy subjects. At the lowest dose of 10 mg (n=12), subjects with BHV-7000 concentrations ≥EC50 (based on preclinical maximal electroshock seizure (MES) models) showed mean increases in EEG spectral power in beta and gamma bands that were not observed in the group of subjects with drug concentrations < EC50 [FIGURE 1a and 1b]. These changes in beta and gamma band activity were consistent with those previously reported for other ASMs (Biondi et al. 2022). At the highest dose of 50 mg (n=11), increases in EEG spectral power were observed across all spectral bands and distributed over all cortical brain regions [FIGURE 2]. In addition to the dose-dependent observations, the time course of the increase in EEG spectral power in the 50mg dose group corresponded to the known pharmacokinetic (PK) profile of BHV-7000. Biohaven expects to present the additional details and analyses from this EEG study at upcoming scientific meetings.
BHV-7000 was well tolerated in the exploratory EEG study and the safety profile was consistent with the previously reported safety data from the Phase 1 SAD/MAD trial completed to date in healthy volunteers [FIGURE 3]. BHV-7000 has been administered at single doses of up to 100 mg and multiple doses of up to 80 mg daily without significant CNS adverse events commonly associated with other ASMs, including a markedly lower incidence of somnolence, speech disorder and memory impairment.
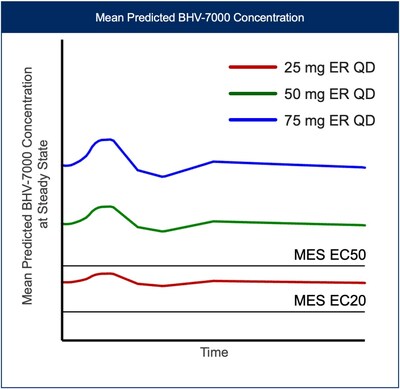
Based on the results from the EEG study and preliminary safety profile in SAD/MAD trials, along with PK data from a new once-daily extended-release (ER) formulation, Biohaven plans on exploring three oral doses of BHV-7000 (once daily 25 mg ER, once daily 50 mg ER, and once daily 75 mg ER) in the Phase 3 focal epilepsy program. This dosing approach with a Kv7 activator will allow for assessment of distinct target concentrations over a wide range, above and below projected efficacious EC50 drug concentrations [FIGURE 4], not previously feasible with drugs in this class. No meaningful food effect was observed in the Phase 1 SAD/MAD trial using BHV-7000 in its standard release formulation.
Vlad Coric, M.D., Chairman and Chief Executive Officer of Biohaven, commented, "The EEG results presented are an important milestone after almost a decade of effort by the ion channel discovery team, led by Drs. Michael Bozik and Steven Dworetzky, to develop a highly selective Kv7.2/7.3 activator with a best-in-class drug profile. The EEG data confirms central nervous system target engagement as measured by dose-dependent changes in spectral power in expected frequency bands and over the entire head. These EEG results along with the safety data from the Phase 1 studies and our formulation group's development of a once-daily extended-release drug formulation of BHV-7000, allow us to advance to pivotal trials later this year. We are excited about evaluating the efficacy of this highly selective Kv7.2/7.3 activator in epilepsy, mood disorders (including bipolar disorder) and other diseases as the profile of BHV-7000 represents a potential paradigm shift for this mechanism of action."
About BHV-7000
BHV-7000, the lead asset from Biohaven's Kv7 platform, is a novel and selective activator of Kv7.2/Kv7.3, a key ion channel involved in neuronal signaling and in regulating the hyperexcitable state, that Biohaven is developing for the treatment of epilepsy and mood disorders. BHV-7000 was rationally developed as a potent activator of heteromeric Kv7.2/7.3 potassium channels, the molecular substrate that underlies the M-current (IKM). BHV-7000 is highly differentiated from ezogabine (known as retigabine in
About Biohaven
Biohaven is a global clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of life-changing therapies to treat a broad range of rare and common diseases. Biohaven's experienced management team brings with it a historical track record of delivering new drug approvals for products for diseases such as migraine, depression, bipolar disorder and schizophrenia. Biohaven is advancing a pipeline of therapies for diseases with little or no treatment options, leveraging its proven drug development capabilities and proprietary platforms, including Kv7 ion channel modulation for epilepsy and neuronal hyperexcitability, glutamate modulation for obsessive-compulsive disorder and spinocerebellar ataxia, myostatin inhibition for neuromuscular diseases, and brain-penetrant TYK2/JAK1 inhibition for immune-mediated brain disorders. Biohaven's portfolio of early- and late-stage product candidates also includes discovery research programs focused on TRPM3 channel activation for neuropathic pain, CD-38 antibody recruiting, bispecific molecules for multiple myeloma, antibody drug conjugates (ADCs), and targeted extracellular protein degrader platform technology (MoDEs™ platform) with potential application in neurological disorders, cancer, and autoimmune diseases. For more information, visit www.biohaven.com.
About Epilog
Epilog is a brand of clouds of care NV, an ISO 13485:2016 and ISO 27001:2013-certified CNS marketplace. Epilog's clinical and technical EEG expertise in epileptiform disorders provides unique EEG-based insights into brain functioning. With CE-marked and FDA-cleared applications for clinicians and tailor-made solutions for clinical trials and research, Epilog is on a mission to optimize epilepsy care as part of the clouds of care CNS portfolio.
For more information, visit www.epilog.care.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the Food and Drug Administration; the timing and outcome of expected regulatory filings; complying with applicable
MoDEs is a trademark of Biohaven Therapeutics Ltd.
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-announces-positive-data-from-its-exploratory-electroencephalogram-eeg-biomarker-study-of-bhv-7000-completion-of-once-daily-formulation-development-and-plan-to-initiate-phase-3-pivotal-studies-301917174.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-announces-positive-data-from-its-exploratory-electroencephalogram-eeg-biomarker-study-of-bhv-7000-completion-of-once-daily-formulation-development-and-plan-to-initiate-phase-3-pivotal-studies-301917174.html
SOURCE Biohaven Ltd.