BeiGene Presents Results from SEQUOIA Trial of BRUKINSA (zanubrutinib) in First-Line Chronic Lymphocytic Leukemia at the 63rd ASH Annual Meeting
BeiGene announced promising results from the interim analysis of the Phase 3 SEQUOIA trial for BRUKINSA in treatment-naïve chronic lymphocytic leukemia (CLL), showing superior progression-free survival (PFS) compared to bendamustine plus rituximab. The 24-month PFS rate for BRUKINSA was 85.5%, significantly higher than 69.5% for the control group. Consistent safety results were noted, with lower rates of adverse events like atrial fibrillation. Preliminary data for BRUKINSA combined with venetoclax in high-risk CLL patients also showed high efficacy and safety.
- BRUKINSA demonstrated superior PFS with a 24-month rate of 85.5% compared to 69.5% for bendamustine plus rituximab.
- The trial showed consistent efficacy across high-risk subgroups, including patients with del(11q) and unmutated IGHV.
- BRUKINSA exhibited a favorable safety profile, with lower rates of atrial fibrillation and serious adverse events.
- Limited overall survival data, with 24-month survival rates being similar between arms.
- Preliminary results in Cohort 3 show only early efficacy and safety data without longer follow-up information.
BRUKINSA demonstrated superiority in progression-free survival over chemoimmunotherapy as a first-line treatment for patients with chronic lymphocytic leukemia
Consistent efficacy was observed across high-risk patient subgroups
Safety results were generally consistent with its previously reported profile
Preliminary safety and efficacy data from Cohort 3 of BRUKINSA in combination with venetoclax for patients with del(17p) or TP53 mutations were reviewed at ASH
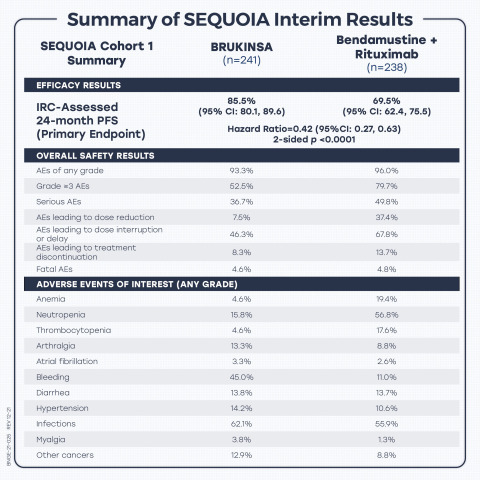
Summary of results from the interim analysis of Phase 3 SEQUOIA trial of BRUKINSA (zanubrutinib) in patients with treatment-naïve chronic lymphocytic leukemia. (Graphic: Business Wire)
“In the positive SEQUOIA trial, BRUKINSA delivered the therapeutic promise of a selective BTK inhibitor as a frontline treatment for CLL patients, with demonstrated superiority over chemoimmunotherapy. These robust data, along with the results from our previously reported Phase 3 ALPINE trial, strengthen our belief that BRUKINSA could become an important new treatment option for patients with CLL,” remarked
“Compared to chemoimmunotherapy, BRUKINSA demonstrated superior PFS benefit for CLL patients receiving frontline treatment, including those harboring high-risk characteristics, such as unmutated IGHV status and del(11q),” said
For more information on BeiGene’s clinical program and company updates, please visit BeiGene’s virtual booth at this year’s ASH Annual Meeting at http://www.beigenevirtualexperience.com.
SEQUOIA Cohort 1: BRUKINSA vs. B+R in TN CLL Patients Without del (17p)
Oral Presentation; Abstract #396; Plain language summary available at https://www.beigene.com/pls/ash2021/sequoia
A total of 479 patients with TN CLL whose tumor did not exhibit del(17p) were enrolled in Cohort 1 of the SEQUOIA trial, with 241 patients randomized to receive BRUKINSA (Arm A) and 238 patients randomized to receive B+R (Arm B). Patient characteristics were balanced between the two arms, with more than
The primary endpoint of the SEQUOIA trial is progression-free survival (PFS) per independent review committee (IRC) assessment in the randomized Cohort 1.
At the interim analysis, with a median follow-up of 26.15 months, BRUKINSA demonstrated superiority in PFS over B+R, as assessed by IRC. Results included:
-
The 24-month PFS rate was
85.5% (95% CI: 80.1, 89.6) in Arm A, compared to69.5% (95% CI: 62.4, 75.5) in Arm B, with a hazard ratio (HR) of 0.42 (95% CI: 0.27, 0.63), p < 0.0001; - PFS benefit was consistently observed across key patient subgroups, including patients with del(11q), unmutated IGHV status, Binet stage C, and bulky disease; and
-
Overall survival (OS) results were early, and at 24 months, OS probability was similar between two arms, with
94.3% (95% CI: 90.4, 96.7) in Arm A and94.6% (95% CI: 90.6, 96.9) in Arm B.
Safety analysis was based on 240 patients in Arm A and 227 patients in Arm B who received at least one dose of respective treatment. BRUKINSA was generally well tolerated with a safety profile consistent with its broad clinical program, including a low rate of atrial fibrillation. Results included:
-
224 patients (
93.3% ) in Arm A experienced at least one adverse event (AE) of any grade, with the most common (≥12% ) being contusion (19.2% ), upper respiratory tract infection (17.1% ), neutropenia (15.4% ), diarrhea (13.8% ), and arthralgia (13.3% ); -
In comparison, 218 patients (
96.0% ) in Arm B experienced at least one AE of any grade, with the most common (≥12% ) being neutropenia (56.8% ), nausea (32.6% ), pyrexia (26.4% ), rash (19.4% ), anemia (18.9% ), constipation (18.9% ), infusion-related reaction (18.9% ), fatigue (15.9% ), vomiting (14.5% ), thrombocytopenia (13.7% ), and diarrhea (13.2% ); -
126 patients (
52.5% ) in Arm A experienced at least one Grade ≥3 AE, compared to 181 patients (79.7% ) in Arm B, with the most common in both arms being neutropenia (11.3% in Arm A vs.51.1% in Arm B) and thrombocytopenia (1.7% in Arm A vs.7.0% in Arm B); -
88 patients (
36.7% ) in Arm A experienced at least one serious AE, compared to 113 patients (49.8% ) in Arm B; -
AEs leading to dose reduction, interruption or delay, and discontinuation occurred in 18 patients (
7.5% ), 111 patients (46.3% ), and 20 patients (8.3% ), respectively, in Arm A, compared to 84 patients (37.4% ), 154 patients (67.8% ), and 31 patients (13.7% ), respectively, in Arm B; -
Fatal AEs were reported in 11 patients (
4.6% ) in Arm A, compared to 11 patients (4.8% ) in Arm B; -
AEs of interest of any grade included anemia (Arm A vs. Arm B:
4.6% vs.19.4% ), arthralgia (13.3% vs.8.8% ), atrial fibrillation (3.3% vs.2.6% ), bleeding (45.0% vs.11.0% ), diarrhea (13.8% vs.13.7% ), hypertension (14.2% vs.10.6% ), infections (62.1% vs.55.9% ), myalgia (3.8% vs.1.3% ), neutropenia (15.8% vs.56.8% ), other cancers (12.9% vs.8.8% ), and thrombocytopenia (4.6% vs.17.6% ).
In addition, efficacy results with an extended follow-up from Cohort 2 (Arm C) of BRUKINSA as a monotherapy in patients with del(17p) were reported at ASH. With a median follow-up of 30.5 months, the 24-month PFS rate was
Summary of SEQUOIA Cohort 1 Interim Analysis
SEQUOIA Cohort 1 Summary |
BRUKINSA (n=241) |
|
Bendamustine + Rituximab (n=238) |
|
Efficacy Results |
||||
IRC-Assessed 24-month PFS (Primary Endpoint) |
( |
|
( |
|
2-sided p <0.0001 |
||||
Overall Safety Results |
||||
AEs of any grade |
|
|
|
|
Grade ≥3 AEs |
|
|
|
|
Serious AEs |
|
|
|
|
AEs leading to dose reduction |
|
|
|
|
AEs leading to dose interruption or delay |
|
|
|
|
AEs leading to treatment discontinuation |
|
|
|
|
Fatal AEs |
|
|
|
|
Adverse Events of Interest (Any Grade) |
||||
Anemia |
|
|
|
|
Neutropenia |
|
|
|
|
Thrombocytopenia |
|
|
|
|
Arthralgia |
|
|
|
|
Atrial fibrillation |
|
|
|
|
Bleeding |
|
|
|
|
Diarrhea |
|
|
|
|
Hypertension |
|
|
|
|
Infections |
|
|
|
|
Myalgia |
|
|
|
|
Other cancers |
|
|
|
|
SEQUOIA Cohort 3 (Arm D): BRUKINSA + Venetoclax in TN CLL Patients with del(17p) and/or TP53 Mutations
Oral Presentation; Abstract #67
Cohort 3 of SEQUOIA was designed to examine the hypothesis that the addition of venetoclax to BRUKINSA can drive tumors into deeper remission. Building on the demonstrated efficacy and safety of BRUKINSA in Cohort 2, Cohort 3 is planned to enroll approximately 80 patients with TN CLL whose tumor exhibits del(17p) or TP53 mutations, with key endpoints being safety, overall response rate (ORR), PFS, and duration of response (DoR). These patients will receive BRUKINSA treatment at 160 mg twice daily for three months, followed by combination treatment of BRUKINSA at the same dosing and venetoclax with a ramp-up dosing to 400 mg once daily for 12 to 24 cycles until progressive disease, unacceptable toxicity, or confirmed undetectable measurable residual disease (uMRD).
“Unfavorable prognosis is often seen in CLL patients with del(17p) or pathogenic TP53 variants, even in the front-line setting. While the follow-up in Cohort 3 was relatively short, the high response rate and the deepened responses observed among those treated for longer periods suggested the potential of BRUKINSA in combination with venetoclax in these high-risk CLL patients. The combination treatment also appeared generally well tolerated,” commented
At the data cutoff on
With a short median follow-up of 12.0 months, a high ORR was observed in the 36 patients who had at least one post-baseline response evaluation by the data cutoff date. Preliminary efficacy results per investigator assessment included:
-
Of the 14 patients who received combination treatment for more than 12 months, five patients (
36% ) achieved a confirmed complete response (CR) or CR with incomplete bone marrow recovery (CRi) in a bone marrow assessment and four additional patients met the criteria for CR or CRi but not confirmed in bone marrow assessment due to COVID-19 restrictions; and -
In all 36 patients evaluable for efficacy, the ORR was
97.2% (95% CI: 85.5, 99.9) and the CR/CRi rate was13.9% (all CRs or CRis were in patients who received combination treatment for more than 12 months).
With a median follow-up of 7.9 months, safety results in all 49 enrolled patients included:
-
40 patients (
81.6% ) experienced at least one AE of any grade, with the most common (≥12% ) being infections (16.3% ), neutropenia (14.3% ), bruising (12.2% ), diarrhea (12.2% ), minor bleeding (12.2% ), and nausea (12.2% ); -
16 patients (
32.7% ) experienced at least one Grade ≥3 AE and four patients (8.2% ) experienced at least one serious AE; -
AEs leading to dose interruption, dose reduction, and treatment discontinuation occurred in 10 patients (
20.4% ), no patients (0.0% ), and one patient (2.0% ), respectively; and -
One patient (
2.0% ) experienced a fatal AE.
With a median follow-up of 13.5 months, safety results in the 34 patients who received combination treatment included:
-
29 patients (
85.3% ) experienced at least one AE of any grade, with the most common (≥12% ) being infections (23.5% ), neutropenia (20.6% ), diarrhea (14.7% ), fatigue (14.7% ), nausea (14.7% ), and bruising (11.8% ); -
13 patients (
38.2% ) experienced at least one Grade ≥3 AE and three patients (8.8% ) experienced at least one serious AE; and -
AEs leading to dose interruption occurred in 10 patients (
29.4% ), with no AEs leading to dose reduction or treatment discontinuation.
About SEQUOIA
SEQUOIA is a randomized, multicenter, global Phase 3 trial (NCT03336333) designed to evaluate the efficacy and safety of BRUKINSA compared to B+R in patients with TN CLL or SLL. The trial consists of three cohorts:
- Cohort 1 (n=479): randomized 1:1 to receive BRUKINSA (n=241) or B+R (n=238) until disease progression or unacceptable toxicity, in patients not harboring del(17p); data from this group comprise the primary endpoint;
- Cohort 2 (n=110): patients with del(17p) receiving BRUKINSA as a monotherapy; and
- Cohort 3 (enrollment ongoing): patients with del(17p) or pathogenic TP53 variant receiving BRUKINSA in combination with venetoclax.
Patients with del(17p) were not randomized to B+R, as they experience poor clinical outcomes and poor response to chemoimmunotherapy. The primary endpoint of the trial is IRC-assessed PFS. Secondary endpoints include investigator-assessed PFS, IRC- and investigator-assessed overall response rate (ORR), overall survival (OS), PFS and ORR in patients with del(17p), and safety.
Cohort 2 (Arm C), representing high-risk patients treated with BRUKINSA monotherapy, was previously presented at the
About Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in adults, with a global incidence of approximately 114,000 new cases in 2017.1,2 CLL affects white blood cells or lymphocytes in the bone marrow.1 Proliferation of cancer cells (leukemia) in the marrow result in reduced ability to fight infection and spread into the blood, which affects other parts of the body including the lymph nodes, liver and spleen.1,3 The BTK pathway is a known route that signals malignant B cells and contributes to the onset of CLL.4 Small lymphocytic lymphoma (SLL) is a non-Hodgkin’s lymphoma affecting the B-lymphocytes of the immune system, which shares many similarities to CLL but with cancer cells found mostly in lymph nodes.5
About BRUKINSA
BRUKINSA is a small molecule inhibitor of Bruton’s tyrosine kinase (BTK) discovered by
BRUKINSA has received 12 approvals covering 40 countries and regions:
-
For the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy (
United States ,November 2019 )*; -
For the treatment of MCL in adult patients who have received at least one prior therapy (
China ,June 2020 )**; -
For the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adult patients who have received at least one prior therapy (
China ,June 2020 )**; -
For the treatment of relapsed or refractory MCL (
United Arab Emirates ,February 2021 ); -
For the treatment of Waldenström’s macroglobulinemia (WM) in adult patients (
Canada ,March 2021 ); -
For the treatment of adult patients with WM who have received at least one prior therapy (
China ,June 2021 )**; -
For the treatment of MCL in adult patients who have received at least one prior therapy (
Canada ,July 2021 ); -
For the treatment of MCL in adult patients who have received at least one prior therapy (
Chile ,July 2021 ); -
For the treatment of adult patients with MCL who have received at least one previous therapy (
Brazil ,August 2021 ); -
For the treatment of adult patients with WM (
United States ,August 2021 ); -
For the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one anti-CD20-based regimen (
United States ,September 2021 )*; -
For the treatment of adult patients with MCL who have received at least one previous therapy (
Singapore ,October 2021 ); -
For the treatment of MCL in patients who have received at least one prior therapy (
Israel ,October 2021 ); -
For the treatment of adult patients with WM who have received at least one prior therapy, or in first line treatment for patients unsuitable for chemo-immunotherapy (
Australia ,October 2021 ); -
For the treatment of adult patients with MCL who have received at least one prior therapy (
Australia ,October 2021 ); -
For the treatment of adult patients with MCL who have received at least one previous therapy (
Russia ,October 2021 ); -
For the treatment of adult patients with MCL who have received at least one previous therapy (
Saudi Arabia ,November 2021 ); and -
For the treatment of adult patients with WM who have received at least one prior therapy or first-line treatment of patients unsuitable for chemo-immunotherapy (
European Union plusIceland andNorway ,November 2021 ).
To date, more than 20 marketing authorization applications have been submitted for BRUKINSA for various indications.
* This indication was approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
** This indication was approved under conditional approval. Complete approval for this indication may be contingent upon results from ongoing randomized, controlled confirmatory clinical trials.
IMPORTANT
Warnings and Precautions
Hemorrhage
Fatal and serious hemorrhagic events have occurred in patients with hematological malignancies treated with BRUKINSA monotherapy. Grade 3 or higher hemorrhage including intracranial and gastrointestinal hemorrhage, hematuria and hemothorax have been reported in
Bleeding events have occurred in patients with and without concomitant antiplatelet or anticoagulation therapy. Co-administration of BRUKINSA with antiplatelet or anticoagulant medications may further increase the risk of hemorrhage.
Monitor for signs and symptoms of bleeding. Discontinue BRUKINSA if intracranial hemorrhage of any grade occurs. Consider the benefit-risk of withholding BRUKINSA for 3-7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
Infections
Fatal and serious infections (including bacterial, viral, or fungal) and opportunistic infections have occurred in patients with hematological malignancies treated with BRUKINSA monotherapy. Grade 3 or higher infections occurred in
Consider prophylaxis for herpes simplex virus, pneumocystis jiroveci pneumonia and other infections according to standard of care in patients who are at increased risk for infections. Monitor and evaluate patients for fever or other signs and symptoms of infection and treat appropriately.
Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (
Monitor complete blood counts regularly during treatment and interrupt treatment, reduce the dose, or discontinue treatment as warranted. Treat using growth factor or transfusions, as needed.
Second Primary Malignancies
Second primary malignancies, including non-skin carcinoma, have occurred in
Cardiac Arrhythmias
Atrial fibrillation and atrial flutter were reported in
Embryo-Fetal Toxicity
Based on findings in animals, BRUKINSA can cause fetal harm when administered to a pregnant woman. Administration of zanubrutinib to pregnant rats during the period of organogenesis caused embryo-fetal toxicity including malformations at exposures that were 5 times higher than those reported in patients at the recommended dose of 160 mg twice daily. Advise women to avoid becoming pregnant while taking BRUKINSA and for 1 week after the last dose. Advise men to avoid fathering a child during treatment and for 1 week after the last dose.
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Adverse reactions
The most common adverse reactions, including laboratory abnormalities, in ≥
Drug Interactions
CYP3A Inhibitors: When BRUKINSA is co-administered with a strong CYP3A inhibitor, reduce BRUKINSA dose to 80 mg once daily. For coadministration with a moderate CYP3A inhibitor, reduce BRUKINSA dose to 80 mg twice daily.
CYP3A Inducers: Avoid coadministration with moderate or strong CYP3A inducers.
Specific Populations
Hepatic Impairment: The recommended dose of BRUKINSA for patients with severe hepatic impairment is 80 mg orally twice daily.
Please see full
BeiGene Oncology
About
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws, including statements regarding the results from the interim analysis of the Phase 3 SEQUOIA trial, the potential clinical benefits and advantages of BRUKINSA,
References
-
American Cancer Society . Cancer Facts & Figures 2021.Atlanta ;American Cancer Society ; 2021. Available here: Cancer Facts and Figures 2021. - Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. JAMA Oncol. 2019;5(12):1749-1768.
-
National Cancer Institute . Chronic Lymphocytic Leukemia Treatment (PDQ®)–Patient Version. Available here: Chronic Lymphocytic Leukemia Treatment (PDQ®)–Patient Version. - Haselager MV et al. Proliferative Signals in Chronic Lymphocytic Leukemia; What Are We Missing? Front Oncol. 2020; 10: 592205.
-
Cancer Support Community . Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Available here: https://www.cancersupportcommunity.org/chronic-lymphocytic-leukemiasmall-lymphocytic-lymphoma.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211212005018/en/
Investor Contact
+86 10-5895-8058
ir@beigene.com
Media Contact
+1 857-302-7596
media@beigene.com
Source:
FAQ
What were the results of the SEQUOIA trial for BRUKINSA (BGNE)?
How does BRUKINSA compare to chemoimmunotherapy?
What safety profile does BRUKINSA have?
What is the significance of the 24-month PFS rate in the trial?







