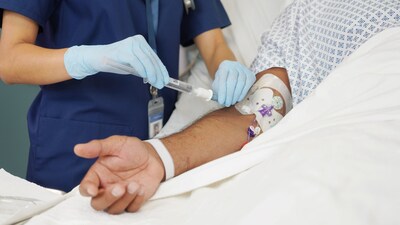
BD Advances Prefilled Flush Syringe with Integrated 'Scrub-the-Hub' Innovation
BD PosiFlush™ SafeScrub Streamlines Clinical Workflow for Disinfecting and Flushing IV Catheters to Help Improve Patient Care
IV lines are an integral part of the care process for many patients with up to 90 percent of hospitalized patients receiving an IV.1 Yet the improper care and maintenance of these devices is one of the leading sources of catheter-related bloodstream infections (CRBSIs).2 While the clinical practice guidelines are clear on the importance of disinfecting a needle-free connector before every access — a process commonly called "scrub the hub" — compliance has been reported to be as low as 10 percent.2
BD PosiFlush™ SafeScrub is a prefilled saline flush syringe that features an active disinfection unit built right into the tip cap of the syringe that is demonstrated via in vitro testing to significantly reduce microbial growth associated with CRBSIs.3 By integrating the scrubbing device into the syringe unit, the device is designed to more easily enable compliance to "scrub the hub" guidelines and reduce touch contamination. This design simplifies steps and streamlines workflow for nurses, while providing a tool to help protect patients.
"Health care facilities are continuing to feel the burden from the pandemic with high rates of nursing turnover and shortages," said Dr. Nathan Gilmore, chief of service critical care at Hoag Hospital in
As the global leader in vascular access solutions, BD is committed to advancing the standard of care for IV therapy for patients and health care providers. BD PosiFlush™ SafeScrub received clearance and was introduced to select customers in October 2022. The innovation is part of the company's vascular access management portfolio and further drives the BD "One-Stick Hospital Stay" vision by helping to reduce complications, including infections, and extend catheter dwell times through proper IV care and maintenance, which may result in improved clinical outcomes. Optimal maintenance of the IV line helps to reduce the risk of complications so it does not have to be replaced and lasts throughout a patient's hospital stay. This is the third pillar of the "one-stick stay" vision that also includes first, reducing unnecessary needlesticks by choosing the right vascular access device and placing it successfully the first time and, second, using one IV line as a single access point for required therapies and blood draws.
"We continue to hear the need from health care professionals for new solutions that help make medication delivery safer and simpler," said Eric Borin, worldwide president for Medication Delivery Solutions at BD. "Our latest innovation in scrub and flush technology is designed to support nurses by simplifying workflow and advancing evidence-based best practices in catheter care so they can continue to focus their time where it matters most – on patient care."
Learn more about BD PosiFlush™ SafeScrub.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 77,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts: | |
Media: | Investors: |
Alyssa Kretlow | Francesca DeMartino |
Associate Director, Corporate Communications | SVP, Head of Investor Relations |
551.238.4391 | 201.847.5743 |
References:
1 Helm RE, Klausner JD, Klemperer JD, et al. Accepted but unacceptable: peripheral IV catheter failure. Infus Nurs Society. 2015;38(3):189-203.
2 Moureau NL, Flynn J. Disinfection of Needleless Connector Hubs: Clinical Evidence Systematic Review. Nurs Res Pract. 2015;2015:796762.
3 BD Inc. Data on file. In vitro test results may not be predictive of clinical performance. Demonstrated reduction of the most common causative agents of CRBSI including Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, Candida glabrata and Candida albicans.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/bd-advances-prefilled-flush-syringe-with-integrated-scrub-the-hub-innovation-301831173.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/bd-advances-prefilled-flush-syringe-with-integrated-scrub-the-hub-innovation-301831173.html
SOURCE BD (Becton, Dickinson and Company)