BriaCell Confirms 100% Resolution of Lung Metastasis with Bria-OTS™
BriaCell Therapeutics (Nasdaq: BCTX) has confirmed complete resolution of lung metastasis in their Phase 1/2a Bria-OTS study. The breakthrough was observed in a 78-year-old woman with hormone receptor positive (HR+) metastatic breast cancer who had previously failed multiple therapies.
The patient, enrolled on November 21, 2024, received Bria-OTS intradermal injections every 2 weeks for six weeks (4 doses), followed by dosing every 3 weeks. The treatment resulted in complete resolution of lung metastasis at 2 months, confirmed at 4 months follow-up, with stable disease maintained elsewhere.
The patient entered the study with extensive metastases in bone, lymph node, and lung. This response was achieved with the lowest dose level in the trial, demonstrating promising activity of the Bria-OTS platform as monotherapy. The Phase 1/2a dose escalation study (NCT06471673) will evaluate Bria-OTS both as monotherapy and in combination with an immune checkpoint inhibitor.
BriaCell Therapeutics (Nasdaq: BCTX) ha confermato la completa scomparsa delle metastasi polmonari nel loro studio di Fase 1/2a Bria-OTS. La svolta è stata osservata in una donna di 78 anni con cancro al seno metastatico positivo ai recettori ormonali (HR+) che aveva precedentemente fallito molteplici terapie.
La paziente, arruolata il 21 novembre 2024, ha ricevuto iniezioni intradermiche di Bria-OTS ogni 2 settimane per sei settimane (4 dosi), seguite da somministrazioni ogni 3 settimane. Il trattamento ha portato alla completa scomparsa delle metastasi polmonari dopo 2 mesi, confermata al follow-up a 4 mesi, con malattia stabile nelle altre sedi.
La paziente è entrata nello studio con metastasi estese a ossa, linfonodi e polmoni. Questa risposta è stata ottenuta al livello di dose più basso del trial, dimostrando un’attività promettente della piattaforma Bria-OTS come monoterapia. Lo studio di dose escalation di Fase 1/2a (NCT06471673) valuterà Bria-OTS sia come monoterapia sia in combinazione con un inibitore del checkpoint immunitario.
BriaCell Therapeutics (Nasdaq: BCTX) ha confirmado la resolución completa de las metástasis pulmonares en su estudio de Fase 1/2a Bria-OTS. Este avance se observó en una mujer de 78 años con cáncer de mama metastásico positivo a receptores hormonales (HR+) que había fracasado en múltiples tratamientos previos.
La paciente, inscrita el 21 de noviembre de 2024, recibió inyecciones intradérmicas de Bria-OTS cada 2 semanas durante seis semanas (4 dosis), seguidas de dosis cada 3 semanas. El tratamiento resultó en la resolución completa de las metástasis pulmonares a los 2 meses, confirmada en el seguimiento a los 4 meses, manteniendo la enfermedad estable en otras áreas.
La paciente ingresó al estudio con metástasis extensas en hueso, ganglios linfáticos y pulmón. Esta respuesta se logró con el nivel de dosis más bajo del ensayo, demostrando una actividad prometedora de la plataforma Bria-OTS como monoterapia. El estudio de escalada de dosis de Fase 1/2a (NCT06471673) evaluará Bria-OTS tanto como monoterapia como en combinación con un inhibidor de puntos de control inmunitarios.
BriaCell Therapeutics (나스닥: BCTX)는 1/2a상 Bria-OTS 연구에서 폐 전이 완전 소실을 확인했습니다. 이 획기적인 결과는 다수의 치료에 실패한 호르몬 수용체 양성(HR+) 전이성 유방암을 가진 78세 여성 환자에서 관찰되었습니다.
2024년 11월 21일 등록된 이 환자는 6주간 2주 간격으로 Bria-OTS 피내 주사를 4회 투여받았으며, 이후 3주 간격으로 투여가 이어졌습니다. 치료 결과 폐 전이가 2개월 만에 완전히 소실되었고 4개월 추적 관찰에서도 확인되었으며, 다른 부위의 병변은 안정적으로 유지되었습니다.
이 환자는 뼈, 림프절, 폐에 광범위한 전이가 있는 상태로 연구에 참여했습니다. 이번 반응은 임상 시험에서 가장 낮은 용량 수준에서 나타났으며, Bria-OTS 플랫폼의 단독요법으로서 유망한 효능을 보여주었습니다. 1/2a상 용량 증량 연구(NCT06471673)는 Bria-OTS를 단독요법과 면역관문억제제 병용요법으로 평가할 예정입니다.
BriaCell Therapeutics (Nasdaq : BCTX) a confirmé la résolution complète des métastases pulmonaires dans leur étude de phase 1/2a Bria-OTS. Cette avancée a été observée chez une femme de 78 ans atteinte d’un cancer du sein métastatique hormonosensible (HR+) qui avait précédemment échoué à plusieurs traitements.
La patiente, incluse le 21 novembre 2024, a reçu des injections intradermiques de Bria-OTS toutes les 2 semaines pendant six semaines (4 doses), suivies d’une administration toutes les 3 semaines. Le traitement a entraîné une résolution complète des métastases pulmonaires à 2 mois, confirmée lors du suivi à 4 mois, avec une maladie stable dans les autres localisations.
La patiente est entrée dans l’étude avec des métastases étendues aux os, aux ganglions lymphatiques et aux poumons. Cette réponse a été obtenue avec le niveau de dose le plus faible de l’essai, démontrant une activité prometteuse de la plateforme Bria-OTS en monothérapie. L’étude d’escalade de dose de phase 1/2a (NCT06471673) évaluera Bria-OTS à la fois en monothérapie et en association avec un inhibiteur de point de contrôle immunitaire.
BriaCell Therapeutics (Nasdaq: BCTX) hat in ihrer Phase 1/2a Bria-OTS-Studie die vollständige Rückbildung von Lungenmetastasen bestätigt. Dieser Durchbruch wurde bei einer 78-jährigen Frau mit hormonrezeptorpositivem (HR+) metastasiertem Brustkrebs beobachtet, die zuvor mehrere Therapien nicht erfolgreich durchlaufen hatte.
Die Patientin, die am 21. November 2024 eingeschlossen wurde, erhielt intradermale Injektionen von Bria-OTS alle 2 Wochen über sechs Wochen (4 Dosen), gefolgt von einer Gabe alle 3 Wochen. Die Behandlung führte nach 2 Monaten zu einer vollständigen Rückbildung der Lungenmetastasen, die bei der Nachuntersuchung nach 4 Monaten bestätigt wurde, während die Erkrankung an anderen Stellen stabil blieb.
Die Patientin trat mit ausgedehnten Metastasen in Knochen, Lymphknoten und Lunge in die Studie ein. Diese Reaktion wurde mit der niedrigsten Dosisstufe im Versuch erzielt und zeigt die vielversprechende Wirksamkeit der Bria-OTS-Plattform als Monotherapie. Die Phase 1/2a-Dosiseskalationsstudie (NCT06471673) wird Bria-OTS sowohl als Monotherapie als auch in Kombination mit einem Immun-Checkpoint-Inhibitor evaluieren.
- Complete resolution (100%) of lung metastasis achieved and maintained for 4 months
- Positive clinical response achieved with lowest dose level
- Treatment well-tolerated by patient
- Stable disease maintained in other areas
- Results to single patient case study
- Treatment only partially effective with stable disease elsewhere
Insights
Complete resolution of lung metastasis in HR+ breast cancer patient validates BriaCell's personalized immunotherapy approach, showing promising efficacy at lowest dose.
The confirmed complete resolution of lung metastasis in a metastatic breast cancer patient at 4-month follow-up represents a clinically meaningful outcome that deserves attention. This patient had failed several prior lines of therapy and was receiving the lowest dose level in the Phase 1/2a study, making the response particularly notable.
What's scientifically significant is achieving this response in a hormone receptor positive (HR+) breast cancer patient. HR+ tumors have historically shown responsiveness to immunotherapy approaches compared to triple-negative breast cancer. The complete resolution of a metastatic lesion with immunotherapy alone in this setting is uncommon.
The durability of response (confirmed at 4 months) suggests potential for sustained clinical benefit. In metastatic breast cancer, durable responses are challenging to achieve, particularly in heavily pre-treated patients. The stability of disease elsewhere indicates the therapy is providing systemic benefit beyond the resolved lung lesion.
BriaCell's approach combines personalization with "off-the-shelf" availability - a significant technical innovation attempting to overcome manufacturing challenges that have other personalized immunotherapies. This early signal in their first treated patient provides preliminary validation of their platform technology.
The planned dose escalation and future combination with checkpoint inhibitors could potentially enhance these already promising results. While this single-patient data requires confirmation in larger cohorts, the strong anti-tumor activity at the lowest dose level provides a compelling rationale for continued clinical development.
Early clinical validation of BriaCell's immunotherapy platform showing complete tumor resolution potentially derisks their broader pipeline and technology approach.
This clinical update provides preliminary validation for BriaCell's personalized immunotherapy platform in a market where positive clinical signals can significantly impact company trajectory. The complete resolution of lung metastasis confirmed at 4 months represents an important efficacy signal for their novel Bria-OTS technology.
The response characteristics are particularly noteworthy from a development perspective: efficacy observed at the lowest dose level, in a heavily pre-treated patient, and as monotherapy. This creates multiple pathways to potentially enhance efficacy: dose escalation, patient selection refinement, and planned combination approaches with checkpoint inhibitors.
Strategically, this result partially derisks BriaCell's broader platform technology. Bria-OTS represents their next-generation approach, while their lead candidate Bria-IMT is already advancing in a pivotal Phase 3 study. Success in one program provides technical validation that may apply across their pipeline.
The "personalized off-the-shelf" approach addresses a critical industry challenge: balancing personalization benefits with manufacturing practicality. If validated in larger cohorts, this could represent a competitive advantage in the crowded cancer immunotherapy space.
For a company with a
- (IMAGES BELOW) Complete resolution of lung metastasis confirmed at 4 month follow-up in hormone receptor positive (HR+) breast cancer patient
- Treatment well-tolerated and patient remains on study with stable disease elsewhere
- Sustained clinical response supports Bria-OTS personalized, off-the-shelf immunotherapy approach in Phase 1/2a metastatic breast cancer study
PHILADELPHIA and VANCOUVER, British Columbia, April 24, 2025 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care, confirms the sustained complete resolution of the lung metastasis, first reported in February 2025, two months after initial treatment in the ongoing Phase 1/2 Bria-OTS study. The latest data at four months also demonstrates stable disease elsewhere.
Figure 1: Treatment with Bria-OTS monotherapy resulted in
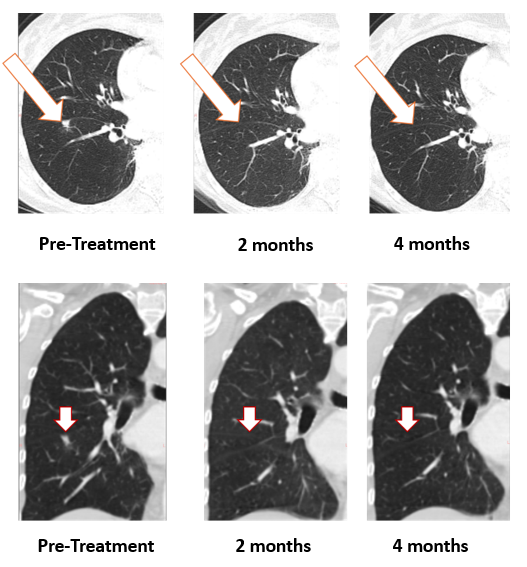
As shown, the lesion in the patient’s right lung is undetectable after two months and confirmed resolved at 4 months. The updated images supersede those previously reported.
The patient, a 78-year-old woman with metastatic breast cancer (hormone receptor positive, HER2 negative), had failed several prior lines of therapy and received the lowest dose level in the Phase 1/2a Bria-OTS study. At enrollment on November 21, 2024, she had extensive metastases including bone, lymph node and lung involvement. Following Bria-OTS intradermal injections every 2 weeks for six weeks (4 total doses), and subsequent dosing every 3 weeks, the lung metastasis completely resolved with stable disease elsewhere. This response is now confirmed and shows the potentially promising activity of the Bria-OTS platform as monotherapy.
“Despite recent advancements with Antibody-drug-conjugates (ADCs) and immune check point inhibitors (CPIs), many patients, including those with HR+ disease, like BriaCell’s first OTS patient, have very few options,” stated Neal S. Chawla MD, Director at the Sarcoma Oncology Center, Santa Monica, Ca., and Principal Investigator for the Bria-OTS study. “We are thrilled with our initial data with single agent Bria-OTS showing rapid and strong anti-tumor activity in an HR+ patient and look forward to continuing this novel approach in patients with MBC, and other cancers.”
“This unprecedented anti-cancer response in the first patient dosed with Bria-OTS is an important milestone for us and provides early validation of BriaCell’s personalized immunotherapy approach,” stated Dr. William V. Williams, BriaCell’s President and CEO.
Bria-OTS is a personalized off-the-shelf immunotherapy, currently under investigation in a Phase 1/2a dose escalation study (ClinicalTrials.gov identifier: NCT06471673) in metastatic recurrent breast cancer. Bria-OTS represents a personalized, next generation, advancement of BriaCell’s lead candidate Bria-IMT™ which is currently in a pivotal Phase 3 study for metastatic breast cancer. The Phase 1/2a clinical trial in metastatic breast cancer is a dose escalation study initially evaluating the safety and efficacy of Bria-OTS as monotherapy and will be followed by Bria-OTS in combination with an immune checkpoint inhibitor.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release include statements regarding: BriaCell continuing the Phase 1/2a Bria-OTS study and reproducing similar results in patients with MBC and other cancers; the use of the Bria-OTS platform as monotherapy; and Bria-OTS’s validation as a personalized immunotherapy approach. Forward-looking statements may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company’s profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
info@briacell.com
Investor Relations Contact:
investors@briacell.com
1 Note that the other white dots in the lungs are blood vessels.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/19a24c7d-e793-4f57-b0df-c1605b7395dc








