Anavex Announces First Entire Clinical Gene Pathway Data of ANAVEX®2-73 from AVATAR Study in Patients with Rett Syndrome
- None.
- None.
Insights
The findings from the AVATAR Rett syndrome trial involving ANAVEX®2-73 present a significant advancement in understanding the therapeutic effects of the drug on neurodevelopmental disorders. The comprehensive analysis of gene expression and identification of pathways affected by the treatment provide a molecular basis for the drug's efficacy. This information is critical for stakeholders, including investors, as it can influence the company's valuation, potential market share and future research and development directions.
From a medical research perspective, the data indicating enhanced mitochondrial energy production and increased expression of genes involved in oxidative phosphorylation and fatty acid metabolism suggest that ANAVEX®2-73 could address underlying metabolic dysfunctions in Rett syndrome. These findings could pave the way for targeted therapies in other neurodevelopmental diseases with similar metabolic components, potentially expanding the drug's applicability and market.
The long-term implications for patients could be profound, as improved understanding and treatment of the metabolic aspects of neurodevelopmental disorders may lead to better clinical outcomes. However, the results must be replicated in larger trials to ensure consistency and reliability before ANAVEX®2-73 can be considered a standard treatment.
The announcement by Anavex Life Sciences Corp. regarding the positive results from their clinical trial for ANAVEX®2-73 in Rett syndrome patients may have a significant impact on the company's financial health. The identification of potential biomarkers of disease pathology and response to treatment not only solidifies the therapeutic's credibility but also enhances its commercial viability.
Investors should note the potential for increased investor confidence and capital inflow, which could drive up the company's stock price. However, the costs associated with further development, regulatory approvals and potential commercialization must be considered when evaluating the company's future financial performance.
Given the specificity of the drug's impact on metabolic pathways, there may also be implications for intellectual property and the possibility of patent extensions, which could further secure long-term revenue streams for Anavex. Nonetheless, the financial analysis must remain cautious until regulatory approvals are secured and the commercialization strategy is clarified.
The therapeutic landscape for neurodevelopmental disorders, particularly Rett syndrome, is limited, with few effective treatments available. The data from Anavex Life Sciences Corp. positions ANAVEX®2-73 as a potential frontrunner in this niche market. The specificity with which the drug targets metabolic and developmental gene pathways could set a new industry standard for treatment efficacy.
As an industry analyst, it's important to evaluate the competitive landscape. The success of ANAVEX®2-73 could disrupt existing treatments and attract partnerships or acquisitions. Biopharmaceutical companies with a focus on neurodevelopmental disorders will likely monitor Anavex's progress closely, as it could signal a shift in research and development focus towards metabolic modulation in these diseases.
Furthermore, the use of comprehensive genomic profiling to inform treatment decisions exemplifies the growing trend towards precision medicine, which is becoming increasingly relevant in the biopharmaceutical industry. Companies that invest in similar approaches may gain a competitive edge.
Significant activation of key developmental and metabolic gene pathways and potential compensatory mechanism with ANAVEX®2-73 in Rett syndrome patients
NEW YORK, Dec. 20, 2023 (GLOBE NEWSWIRE) -- Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease, Parkinson’s disease, Rett syndrome and other central nervous system (CNS) diseases, today announced the first entire clinical gene pathway data from the ANAVEX®2-73-RS-002 AVATAR Rett syndrome trial.
A whole genome and exome analysis comparing drug and placebo in patients with Rett syndrome was performed. We believe the results confirm that Rett syndrome is indeed a neurodevelopmental disorder with a key metabolic component, which can be addressed with therapeutic intervention and is likely relevant for other neurodevelopmental disease indications.
AVATAR study was a randomized, placebo-controlled clinical trial in 33 patients with Rett syndrome which included prespecified biomarkers of response as well as Whole Exome Sequencing (WES) DNA data and full RNA exome expression (RNA-seq) data collection. The study included analysis of gene expression changes in each treatment group as well as analysis of the impact of single nucleotide polymorphisms/variants (SNPs/SNVs) on treatment response.
ANAVEX®2-73 transcriptomics analysis (RNAseq) identified gene networks that are differentially expressed in Rett syndrome patients treated with ANAVEX®2-73 compared to placebo. Patient samples that were analyzed contained on average over 20 million unique reads in both placebo and ANAVEX®2-73 treated patients. Biological relevance of this pool of genes was assessed through pathway analysis and confirmed the impact of ANAVEX®2-73 treatment on pathways involved in neurodevelopmental diseases.
The results highlight many significant differences between active treatment group and placebo, such as the differential expression of several genes in response to ANAVEX®2-73 treatment as shown in the following heatmap figure:
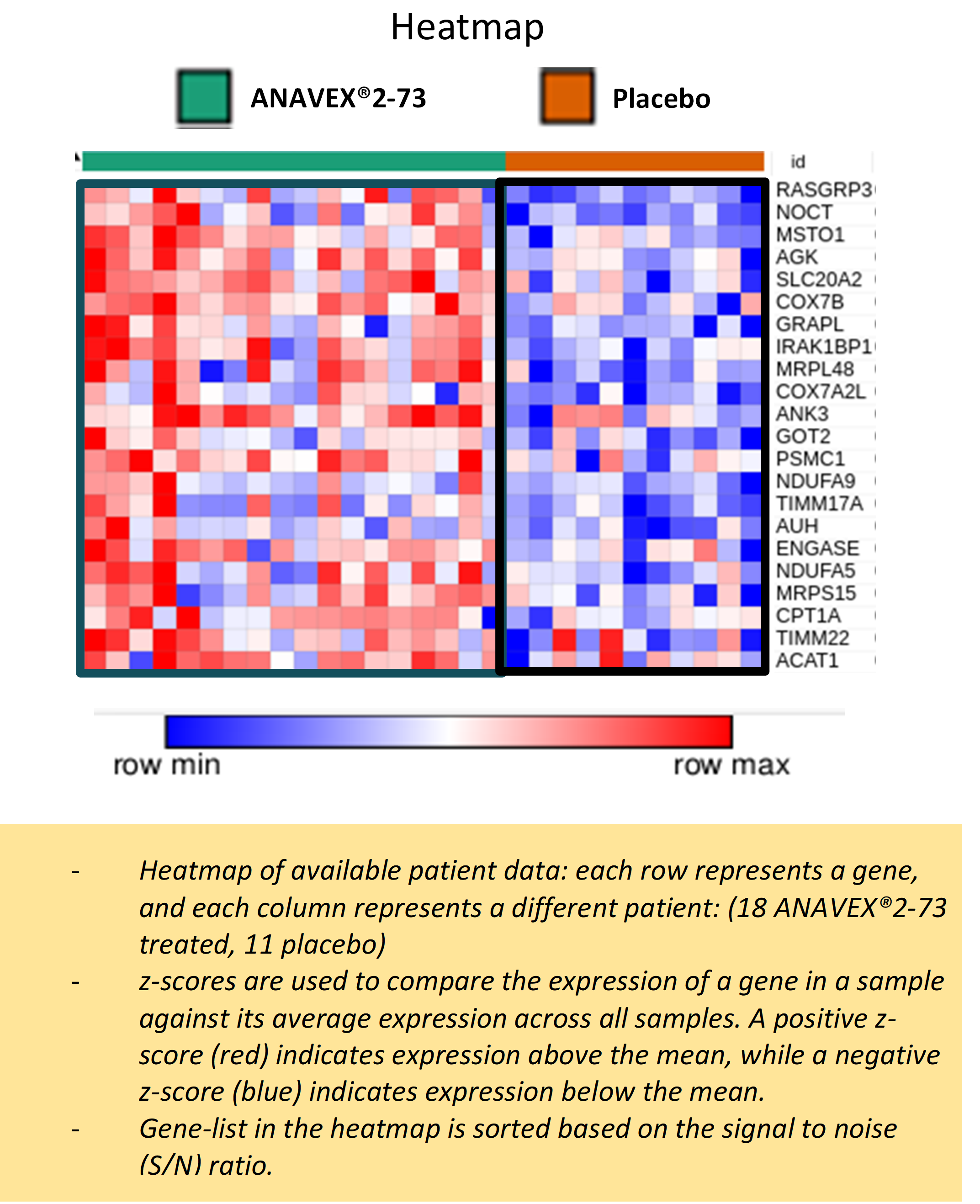
Pathway analysis of the differentially expressed genes suggests ANAVEX®2-73 may, among other functions, correct metabolic alterations in patients with Rett syndrome through enhanced mitochondrial energy production and increased expression of genes involved in oxidative phosphorylation, fatty acid metabolism, developmental signaling pathways, transcription/translation, membrane trafficking, and branched-chain amino acid catabolism as shown in the following table:
| ANAVEX®2-73 Treatment-enriched Pathways | ||
| Pathway Name | Category | p-value |
| Oxidative phosphorylation | Energy Metabolism | 2.84E-04 |
| Valine leucine and isoleucine degradation | Metabolism | 6.85E-04 |
| Adipogenesis | Energy Metabolism | 1.48E-03 |
| Fatty acid degradation | Energy Metabolism | 5.52E-03 |
| Amino acid metabolism | Metabolism | 7.14E-03 |
| Cysteine and methionine metabolism | Metabolism | 8.41E-03 |
| Acyl chain remodeling of phosphatidylserine | Metabolism | 1.43E-02 |
| Mitochondrial protein import | Energy Metabolism | 1.71E-02 |
| α-Linolenic acid metabolism | Metabolism | 1.83E-02 |
| PPAR-α pathway | Signaling | 1.98E-02 |
| Acyl chain remodeling of phosphatidylethanolamine | Metabolism | 2.43E-02 |
| TGF-β signaling pathway | Signaling | 2.49E-02 |
| Deubiquitination | Metabolism | 2.99E-02 |
| NOD1/2 signaling pathway | Signaling | 3.09E-02 |
| Fatty acid β-oxidation | Energy Metabolism | 3.26E-02 |
| Detoxification of reactive oxygen species | Metabolism | 3.26E-02 |
| N-glycan trimming in ER and calnexin/calreticulin cycle | Metabolism | 3.44E-02 |
| miRNAs involvement in the immune response in sepsis | Transcription / Translation | 3.81E-02 |
| RHOV GTPase cycle | Signaling | 3.81E-02 |
| Formation of xylulose-5-phosphate | Metabolism | 4.11E-02 |
| NR1H2 and NR1H3 regulate gene expression linked to gluconeogenesis | Transcription / Translation | 4.11E-02 |
| RHOU GTPase cycle | Signaling | 4.20E-02 |
| Fatty acid metabolism | Energy Metabolism | 4.36E-02 |
| Nucleotide-binding oligomerization domain (NOD) pathway | Transcription / Translation | 4.60E-02 |
| Protein localization | Membrane Trafficking | 4.89E-02 |
| Glycerophospholipid metabolism | Metabolism | 4.89E-02 |
The scope of these detected gene expression changes identified through ANAVEX®2-73 effect may represent additional potential biomarkers of disease pathology and response.
Christopher U Missling, PhD, President and Chief Executive Officer of Anavex, stated, "We believe, this represents an extensive transcriptomics analysis (RNAseq) of a therapeutic agent in patients with Rett syndrome and it is very exciting to witness ANAVEX®2-73’s demonstration of its platform Precision Medicine potential for Rett syndrome with ability to compensate for expression levels of dysregulated developmental and metabolic genes, apparent within the Rett syndrome pathology. We are steadily making progress to learn more about this debilitating disorder and further analysis will be dedicated to evaluating the significance of each identified gene and gene variant and should deepen the understanding of the underlying signaling pathways involved in response to ANAVEX®2-73 treatment in patients with Rett syndrome.”
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders, including Alzheimer's disease, Parkinson's disease, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer. Anavex's lead drug candidate, ANAVEX®2-73 (blarcamesine), has successfully completed a Phase 2a and a Phase 2b/3 clinical trial for Alzheimer's disease, a Phase 2 proof-of-concept study in Parkinson's disease dementia, and both a Phase 2 and a Phase 3 study in adult patients with Rett syndrome. ANAVEX®2-73 is an orally available drug candidate that restores cellular homeostasis by targeting sigma-1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer's disease. ANAVEX®2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective, and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson's Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 for the treatment of Parkinson's disease. ANAVEX®3-71, which targets sigma-1 and M1 muscarinic receptors, is a promising clinical stage drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer's disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid, and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com. You can also connect with the Company on Twitter, Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks set forth in the Company’s most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Anavex Life Sciences Corp. undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development
Toll-free: 1-844-689-3939
Email: info@anavex.com
Investors:
Andrew J. Barwicki
Investor Relations
Tel: 516-662-9461
Email: andrew@barwicki.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/a70a64c6-9af5-487a-91dd-ff744924b733.








