Astria Therapeutics Announces Positive Initial Proof-of-Concept Results from the ALPHA-STAR Phase 1b/2 Trial of STAR-0215 for HAE
- None.
- None.
Insights
The recent announcement by Astria Therapeutics regarding the efficacy of STAR-0215 in reducing monthly attack rates for hereditary angioedema (HAE) patients is a significant development within the biopharmaceutical sector. The reported 90-96% reduction in attack rates and the favorable safety profile underscore the potential of STAR-0215 to become a leading treatment for HAE. The implications of these results for the company are substantial. If the drug continues to demonstrate efficacy and safety in Phase 3 trials, it could lead to a new standard of care, potentially disrupting the current HAE treatment market.
From a medical research perspective, the pharmacokinetics (PK) and pharmacodynamics (PD) data are critical. The consistency of the preliminary PK and PD data with earlier Phase 1a results in healthy subjects is promising. It suggests that the drug's mechanism of action is reliable and could offer sustained benefits to patients. Furthermore, the lack of serious adverse events and the possibility of long-term dosing at intervals of three to six months could improve patient compliance and quality of life.
Investors will likely view the positive trial results of STAR-0215 favorably, as they suggest a high potential for market approval and commercial success. The company's strategic focus on advancing STAR-0215 to Phase 3 trials and the subsequent planning for a Phase 3 pivotal trial, demonstrate a clear path to potential FDA approval and market entry. The financial health of Astria Therapeutics is also a key consideration for stakeholders. With current cash reserves expected to fund operations into mid-2027, the financial runway appears adequate to support the completion of the Q3M Phase 3 pivotal trial without the immediate need for additional financing.
However, it is important to note that the biopharmaceutical industry is highly volatile and the success of clinical trials does not guarantee commercial success. The company's valuation and the stock price will likely be sensitive to future updates on the clinical progress and regulatory feedback. The potential for label expansion to Q6M dosing could further enhance the drug's marketability and provide additional upside to the company's financial prospects.
The potential market impact of STAR-0215 hinges on its ability to offer improved convenience and efficacy over existing HAE treatments. Current therapies often require frequent dosing, which can be burdensome for patients. STAR-0215's longer dosing intervals could position it as a preferred option, provided the Phase 3 trials confirm its safety and efficacy. The market demand for HAE treatments is driven by both the prevalence of the condition and the need for better management options. By potentially reducing the treatment burden, STAR-0215 could capture a significant share of the HAE market.
Additionally, the patient-centric approach, as evidenced by the reduction in attacks requiring rescue medications, could result in high adoption rates among both patients and healthcare providers. This factor, combined with the drug's rapid onset of action, positions Astria Therapeutics to potentially capitalize on unmet needs within the HAE treatment landscape. Market analysts will closely monitor the progression of STAR-0215 through Phase 3 trials and subsequent regulatory reviews to better understand its competitive position and pricing strategy in the context of the broader immunological disease market.
-- STAR-0215 Dosed Once or Twice Over 6 Months Reduced Monthly Attack Rates by 90
-- 92
-- Very Well-Tolerated with No Serious Adverse Events and No Discontinuations --
-- Phase 3 Initiation on Track for Q1 2025, with Top-Line Results Expected by Year End 2026 --
-- Current Cash Expected to Fund Company into Mid-2027 --
-- Conference Call Today at 8:30am ET –
(Photo: Business Wire)
“We are thrilled with these initial results from ALPHA-STAR and believe that STAR-0215 can be a transformative therapy for patients that greatly reduces their disease and treatment burdens,” said Christopher Morabito, M.D., Chief Medical Officer at Astria Therapeutics. “These results give us conviction that we will be able to deliver STAR-0215 once every three and six months, and we look forward to progressing this program into Phase 3 as quickly as possible.”
“The initial results of the ALPHA-STAR trial represent a very exciting step forward in the HAE treatment landscape,” said Marcus Maurer, M.D., Executive Director of the Institute of Allergology at Charite – Universitatsmedizin Berlin. “STAR-0215 has the potential to help patients manage their disease with a mechanism and modality that they trust, but with a substantially improved dosing regimen and the ability to administer without pain. Based on this profile, STAR-0215 has the potential to normalize the lives of people living with HAE.”
"Thanks to the enthusiasm for STAR-0215, the ALPHA-STAR trial enrolled ahead of schedule, enabling us to report these data earlier than originally scheduled,” said Jill C. Milne, Ph.D., Chief Executive Officer. “We believe STAR-0215 has the potential to be the first-choice HAE treatment and I am thankful for the HAE community and the Astria team for helping us to achieve today’s important milestone.”
ALPHA-STAR is a dose-ranging proof-of-concept trial in adults with HAE Type 1 or 2 designed to assess safety, tolerability, efficacy, pharmacokinetics (PK), pharmacodynamics (PD), and quality of life in patients receiving single and multiple doses of STAR-0215 delivered subcutaneously to prevent attacks in HAE. Target enrollment of 16 patients has been achieved and all doses have been administered. All cohorts began with an eight-week run-in period to measure baseline HAE attacks and safety, efficacy, PK, and PD are assessed through 6-months (Day 168) after the last dose received. The initial efficacy and safety data-cut was as of March 13, 2024.
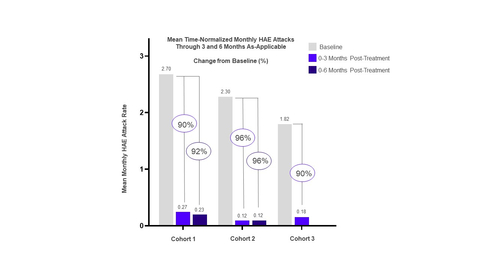
Cohort 1 evaluated a 450 mg dose and all four patients have completed 6 months of follow-up. Efficacy observations compared to baseline through 6 months of follow-up were as follows:
-
92% reduction in monthly attack rate -
96% reduction in moderate and severe attacks -
91% reduction in acute rescue medication use -
50% of patients were attack-free through 3 months of follow-up
Cohort 2 evaluated a 600 mg dose followed by a 300 mg dose three months later, on Day 84. The Company plans to evaluate this dosing regimen in Phase 3. All six patients have completed 3 months of follow up and three patients have completed 6 months of follow-up. Efficacy observations compared to baseline through 6 months of follow-up were as follows:
-
96% reduction in monthly attack rate -
98% reduction in moderate and severe attacks -
94% reduction in acute rescue medication use -
67% of patients were attack-free -
100% of patients were attack-free in the first month after dosing, demonstrating rapid onset of action
Cohort 3 received a 600 mg dose followed by a 600 mg dose one month later, on Day 28. Four of six patients have completed 3 months of follow-up. Efficacy observations compared to baseline through 3 months of follow-up were as follows:
-
90% reduction in monthly attack rate -
100% reduction in moderate and severe attacks -
95% reduction in acute rescue medication use -
50% of patients were attack-free
Preliminary PK and PD data are consistent with Phase 1a data in healthy subjects and consistent with observed efficacy.
STAR-0215 was generally well-tolerated with no serious treatment-emergent adverse events (TEAEs) and no discontinuations. There were two treatment-related TEAEs (both mild), one of which was a case of dizziness and the other a transient injection site reaction (rash). There were no injection site reactions of pain.
After completion of the ALPHA-STAR trial, patients have the opportunity to continue to receive STAR-0215 every three or six months in the long-term open label ALPHA-SOLAR trial. Initial safety and efficacy data from Q3M and Q6M dosing in the ALPHA-SOLAR trial are expected mid-2025.
The observed efficacy, PK, PD, and safety and tolerability profile of STAR-0215 support advancement of STAR-0215 into Phase 3 development. To progress STAR-0215 to market as quickly as possible, the Company plans to focus the Phase 3 program on Q3M dosing initially, immediately followed by a second trial to support label expansion to Q6M. Pending regulatory feedback, the Company expects to start a pivotal Q3M Phase 3 trial in Q1 2025, with top-line results expected by year-end 2026.
The Company expects that its current cash, cash equivalents, and short-term investments of
Webcast Information
The Company will host a webcast today at 8:30am ET. Interested parties may join the webcast via the Investors section of the Astria website, www.astriatx.com or with following the link https://lifescievents.com/event/astriatx/. The webcast will be archived for 90 days.
About Astria Therapeutics:
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development for the treatment of hereditary angioedema. Our second program, STAR-0310, is a monoclonal antibody OX40 antagonist in preclinical development for the treatment of atopic dermatitis. Learn more about our company on our website, www.astriatx.com, or follow us on X and Instagram @AstriaTx and on Facebook and LinkedIn.
About STAR-0215:
STAR-0215 is a monoclonal antibody inhibitor of plasma kallikrein in development for the treatment of HAE. Our goal with STAR-0215 is to provide rapid and sustained HAE attack prevention with a validated mechanism and trusted modality administered every 3 and 6 months. We aim to empower people living with HAE to live life without limitations from their disease. Pending regulatory feedback, we expect to initiate a pivotal Q3M Phase 3 trial in Q1 of 2025 with top-line results expected by year-end 2026.
Forward Looking Statements:
This press release contains forward-looking statements within the meaning of applicable securities laws and regulations including, but not limited to, statements regarding: our expectations regarding the potential significance of the initial results from the Phase 1b/2 ALPHA-STAR clinical trial of STAR-0215, and that the results from such trial will allow us to move directly into a Phase 3 trial of STAR-0215 as a potential treatment for hereditary angioedema (HAE); the expected timing of initiation and design of the planned Phase 3 trials of STAR-0215; the potential therapeutic benefits of STAR-0215 as a treatment for HAE; the potential market impact of STAR-0215 as a treatment for HAE and our vision and goals for the STAR-0215 program; expectations regarding the timing of initiation and planned design of clinical trials for STAR-0310 in AD; expectations regarding the timing and nature of anticipated data from planned trials of STAR-0310; our anticipated cash runway; and our corporate strategy and vision, including the goal to meet the unmet needs of patients with rare and niche allergic and immunological diseases. The use of words such as, but not limited to, “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “goals,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” or "vision," and similar words expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Astria’s current beliefs, expectations and assumptions regarding the future of its business, future plans and strategies, future financial performance, results of pre-clinical and clinical results of the Astria’s product candidates and other future conditions. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including risks and uncertainties related to: changes in applicable laws or regulations; the possibility that we may be adversely affected by other economic, business, and/or competitive factors; risks inherent in pharmaceutical research and development, such as: adverse results in our drug discovery, preclinical and clinical development activities, the risk that the results of preclinical studies may not be replicated in clinical trials, that the preliminary, initial or interim results from clinical trials may not be indicative of the final results, that the results of early stage clinical trials, such as the initial results from the ALPHA-STAR Phase 1b/2 clinical trial, may not be replicated in later stage clinical trials, the risk that we may not be able to enroll sufficient patients in our clinical trials on a timely basis, and the risk that any of our clinical trials may not commence, continue or be completed on time, or at all; decisions made by, and feedback received from, the
Neither Astria, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise, except as required by law. These forward-looking statements should not be relied upon as representing Astria’s views as of any date subsequent to the date hereof.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240325940590/en/
Astria:
Investor Relations and Media:
Elizabeth Higgins
investors@astriatx.com
Source: Astria Therapeutics, Inc.
FAQ
What are the initial results from the ALPHA-STAR Phase 1b/2 clinical trial for STAR-0215 by Astria Therapeutics (ATXS)?
When is the Phase 3 trial initiation for STAR-0215 expected?
How well-tolerated was STAR-0215 in the ALPHA-STAR trial?
What is the expected cash runway for Astria Therapeutics (ATXS) based on the PR?







