Actinium Presents First Ever Data Demonstrating Actimab-A in Combination with Leading Menin Inhibitors Leads to Anti-Tumor Control and Potent Leukemic Cell Killing in Preclinical Acute Myeloid Leukemia Models at the 2024 EHA Congress
Actinium Pharmaceuticals presented new preclinical data at the 2024 European Hematology Association Congress, demonstrating that Actimab-A, in combination with leading menin inhibitors revumenib and ziftomenib, significantly enhances acute myeloid leukemia (AML) cell death compared to monotherapy.
The combination targets KMT2A rearrangements and NMP1 mutations, affecting 10% and 30% of AML patients respectively. Actimab-A showed potent AML cell-killing activity, enhancing cell death in difficult-to-treat KMT2A mutant AML and triggering increased necrosis and cell death in vivo within 72 hours. The anti-tumor effect of the combination was significantly potentiated in xenograft leukemia models.
CEO Sandesh Seth highlighted the broad potential of this combination therapy across various AML treatment settings, noting its promise in both preclinical studies and potential future clinical trials.
- Actimab-A combined with menin inhibitors showed increased AML cell death in preclinical models.
- Significant tumor elimination achieved when Actimab-A is combined with menin inhibitors compared to monotherapy.
- Actimab-A showed potent in vitro AML cell-killing activity compared to non-radio conjugated CD33 antibody lintuzumab.
- The combination resulted in significant anti-tumor effects in xenograft leukemia models in vivo.
- The treatment showed promise within 72 hours of dosing, indicating rapid efficacy.
- Potential for broad application in AML treatment, including frontline, maintenance, and relapsed/refractory settings.
- The data is preclinical, with no guarantee that the results will translate to human clinical trials.
- Potential risks and adverse effects of the combination therapy in humans are not yet known.
- Pending clinical trials and regulatory approval could introduce delays and uncertainties.
- The success of this combination therapy may depend on factors such as patient selection and mutation status.
Insights
Actimab-A combined with menin inhibitors presents a notable advancement in treating acute myeloid leukemia (AML). The
The data presented at the EHA Congress underlines Actimab-A's versatility and efficacy when used in combination therapies, especially given its mutation-agnostic cell-killing mechanism. For a retail investor, this implies potential for broader market application and possibly higher adoption rates, provided further clinical trials affirm these preclinical results. The
However, translating preclinical success to clinical efficacy in humans remains a hurdle. Investors should monitor upcoming clinical trial results closely. Success in these trials could lead to
From a financial perspective, the combination therapy of Actimab-A with menin inhibitors has the potential to significantly increase Actinium Pharmaceuticals' market valuation. Should clinical trials confirm the preclinical findings, Actinium could secure a stronger foothold in the oncology market, particularly for AML treatments.
Actimab-A being used in combination with multiple therapeutics, including menin inhibitors, chemotherapy and FLT3 inhibitors, suggests an expansive commercial pipeline. This could translate to sustained revenue streams and reduced dependency on a single product. The
It's important for investors to consider the current clinical development stages of the menin inhibitors involved. For instance, revumenib has a PDUFA date in September 2024 and successful approval could expedite the combined therapy’s entry into the market, further validating Actinium's strategic direction.
However, it’s important to remain cautious about the inherent risks associated with drug development – high R&D costs and potential delays or failures in clinical trials could impact financial performance.
The presentation at the EHA Congress could potentially position Actinium Pharmaceuticals as a key innovator in the AML treatment arena. Actimab-A's combination with menin inhibitors could address unmet needs in a niche yet significant market segment. The ability to target AML with mutation-agnostic therapies paves the way for Actimab-A to be a backbone therapy across varying treatment protocols.
Actinium’s focus on AML cases with KMT2A and NMP1 mutations opens up a targeted patient demographic that can be precisely marketed to. This strategic approach could enhance the company’s positioning within the oncology market, making it attractive to both healthcare providers and patients due to potentially improved patient outcomes and faster responses.
However, market entry will heavily depend on the outcomes of upcoming clinical trials and subsequent regulatory approvals. Competitive analysis should also not be overlooked – several other companies are developing menin inhibitors, so differentiation and perceived efficacy will play important roles in market penetration.
Retail investors should watch for trends in menin inhibitor approvals and the competitive landscape, as these factors will influence Actimab-A's market potential.
- Actimab-A enhances dose-dependent acute myeloid leukemia cell death in KMT2A sensitive acute myeloid leukemia blasts in combination with leading menin inhibitors
- Combination with leading menin inhibitor demonstrates acute myeloid leukemia cell death and significant tumor elimination not achieved with monotherapy
- Menin combination expands backbone potential of Actimab-A in acute myeloid leukemia that already includes chemotherapy, venetoclax and FLT3 inhibitors
Actimab-A + Menin inhibitor combination results include:
- Actimab-A as a single agent showed potent in vitro AML cell killing activity in both MV-4-11 and MOLM-13 KMT2A mutant cell lines, compared to the non-radio conjugated CD33 antibody lintuzumab (p<0.0001)
- Actimab-A enhanced AML cell death when combined with both revumenib and ziftomenib at all dose levels in difficult to treat KMT2A mutant AML
- The combination of Actimab-A with leading menin inhibitors triggered an acute increase in AML necrosis and cell death in vivo relative to single agent therapy within 72 hours of dosing
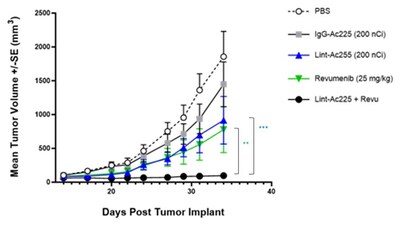
- Anti-tumor effect was significantly potentiated and prolonged when combining Actimab-A with a leading menin inhibitor compared to monotherapies in xenograft leukemia models in vivo (p<0.0024 Actimab-A + menin) as shown in the exhibit below
The Actimab-A + Menin Inhibitor combination presentation can be accessed on the investor relations page of Actinium's website here.
Actimab-A targets CD33, a marker expressed ubiquitously in patients with AML, and is conjugated with the alpha-partible payload Actinium-225. The broad expression of CD33 and the differentiated mutation agnostic cell-killing mechanism of targeted radiotherapy make Actimab-A broadly applicable for combinations with chemotherapy, targeted agents including venetoclax, FLT3 and menin inhibitors, immunotherapies and cellular therapies supporting its potential backbone therapy profile across the AML patient treatment journey.
Sandesh Seth, Actinium's Chairman and CEO, said, "Combining with menin inhibitors is an exciting expansion of the already broad potential of Actimab-A in AML. Across single agent and combination studies, Actimab-A has produced high rates of response, MRD negativity and improved survival in high-risk, relapsed and refractory patients including those with a TP53 mutation and venetoclax failures. The broad expression of CD33 in AML coupled with the potency of Actinium-225 make Actimab-A an ideal agent for treating radiation sensitive AML. We are encouraged by this highly promising initial data and the synergistic potential of Actimab-A with menin inhibitors, which has broad potential across the AML treatment continuum including frontline, maintenance and relapsed/refractory settings. We are eager to continue to study this combination and generate additional data that could support advancing into clinical studies of Actimab-A with menin inhibitors."
Menin inhibitors are a class of drug candidates being developed for patients with AML that have a rearrangement of the KMT2A gene, previously known as the mixed-lineage leukemia (MLL) or mutation of the NPM1 gene. There are multiple menin inhibitors in development for these patients with revumenib (Syndax Pharmaceuticals, Inc.) being most advanced having a PDUFA data of September 2024 and ziftomenib (Kura Oncology, Inc.) enrolling patients in a registration Phase 2 trial. Multiple menin inhibitors are being studied in Phase 1 clinical trials by companies including Johnson & Johnson, Sumitomo Pharma Co., Ltd., Hutchmed, Biomea Fusion, Inc. and BioNova Pharmaceuticals Pvt Ltd.
About Actinium Pharmaceuticals, Inc.
Actinium develops targeted radiotherapies to meaningfully improve survival for people who have failed existing oncology therapies. Advanced pipeline candidates Iomab-B (pre-BLA & MAA (EU)), an induction and conditioning agent prior to bone marrow transplant, and Actimab-A (National Cancer Institute CRADA pivotal development path), a therapeutic agent, have demonstrated potential to extend survival outcomes for people with relapsed and refractory acute myeloid leukemia. Actinium plans to advance Iomab-B for other blood cancers and next generation conditioning candidate Iomab-ACT to improve cell and gene therapy outcomes. Actinium holds more than 230 patents and patent applications including several patents related to the manufacture of the isotope Ac-225 in a cyclotron.
For more information, please visit: https://www.actiniumpharma.com/
Forward-Looking Statements
This press release may contain projections or other "forward-looking statements" within the meaning of the "safe-harbor" provisions of the private securities litigation reform act of 1995 regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update. These statements are based on management's current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Actinium's products and services, performance of clinical research organizations and other risks detailed from time to time in Actinium's filings with the Securities and Exchange Commission (the "SEC"), including without limitation its most recent annual report on form 10-K, subsequent quarterly reports on Forms 10-Q and Forms 8-K, each as amended and supplemented from time to time.
Investors:
investorrelations@actiniumpharma.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-presents-first-ever-data-demonstrating-actimab-a-in-combination-with-leading-menin-inhibitors-leads-to-anti-tumor-control-and-potent-leukemic-cell-killing-in-preclinical-acute-myeloid-leukemia-models-at-the-2024-eha-congr-302173741.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-presents-first-ever-data-demonstrating-actimab-a-in-combination-with-leading-menin-inhibitors-leads-to-anti-tumor-control-and-potent-leukemic-cell-killing-in-preclinical-acute-myeloid-leukemia-models-at-the-2024-eha-congr-302173741.html
SOURCE Actinium Pharmaceuticals, Inc.