Arcturus Therapeutics Reports New Data Demonstrating Neutralizing Antibody Immune Response to the SARS-CoV-2 Omicron Variant from ARCT-154 and ARCT-165 Booster Clinical Trial
Arcturus Therapeutics (NASDAQ: ARCT) announced promising findings from its Phase 1/2 booster trial for mRNA vaccine candidates ARCT-154 and ARCT-165. The study demonstrated 54-fold and 47-fold increases in neutralizing antibody titers against the Omicron variant after administering low doses (5 mcg) at least five months post-initial vaccination. This data supports expanding clinical development aimed at evaluating the candidates as effective COVID-19 booster vaccines. The ongoing trial is being conducted in the U.S. and Singapore.
- Significant 54-fold and 47-fold increases in neutralizing antibody responses for ARCT-154 and ARCT-165 against Omicron.
- Potential for ARCT-154 and ARCT-165 to serve as effective booster vaccines for COVID-19.
- Robust neutralizing antibody responses observed against multiple variants.
- None.
Analysis of sera from Phase 1/2 booster study demonstrates 54- and 47-fold increases in geometric mean neutralizing antibody titers against the Omicron variant at Day 29 for ARCT-154 (5 mcg) and ARCT-165 (5 mcg) respectively
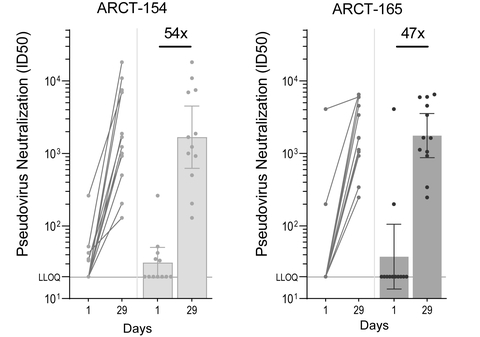
Figure 1: Pseudovirus (Omicron variant, research use) MNT assay results. Antibody titers corresponding to
New data from the Phase 1/2 booster trial, sponsored by Arcturus and currently ongoing in
“Arcturus’ lower dose STARR™ self-amplifying mRNA vaccine technology continues to generate highly encouraging clinical booster data, increasing the platform’s potential to address endemic markets for COVID and other infectious diseases,” said
Twenty four (24) participants, divided equally in the ARCT-154 and ARCT-165 booster groups, received 5 micrograms of ARCT-154 or ARCT-165 following primary vaccination with Comirnaty® at least 5 months earlier. All participants in the booster trial were below 65 years of age at the time of receiving the booster dose. Figure 1 shows the neutralizing antibody geometric mean titers against the Omicron strain for the ARCT-154 and the ARCT-165 groups on Day 29 post-boost compared to pre-boost titer, as measured by an exploratory microneutralization titer (MNT) assay (
About
Founded in 2013 and based in
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the expectations for or likelihood of success of any collaborations, the likelihood of success (including safety and efficacy) of the Company’s platform or pipeline (including ARCT-154 and ARCT-165), the Company’s efforts to develop a vaccine against COVID-19 and therapeutic potential thereof based on the Company’s mRNA therapeutics, the planned initiation, design or completion of clinical trials, the likelihood that the Company will obtain clearance from regulatory authorities to proceed with future planned clinical trials, the likelihood that preclinical or clinical data will be predictive of future clinical results (including with respect to safety, immunogenicity and efficacy), the likelihood that a preliminary, interim or partial data set will be representative of a complete or larger data set, the likelihood that clinical data will be sufficient to support further clinical development, for regulatory approval or will be completed in time to submit an application for regulatory approval within a particular timeframe, the likelihood that a patent will issue from any patent application and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading "Risk Factors" in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks, and trade names in this announcement are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220125005602/en/
IR and Media Contacts
(858) 900-2682
IR@ArcturusRx.com
Kendall Investor Relations
(617) 914-0008
ctanzi@kendallir.com
Source:
FAQ
What are the key findings from Arcturus Therapeutics' recent press release regarding ARCT-154 and ARCT-165?
What is the purpose of the Phase 1/2 booster trial for ARCT-154 and ARCT-165?
Where is the booster trial for ARCT-154 and ARCT-165 being conducted?







