Aquestive Therapeutics Announces Positive Topline PK Results from its Pediatric Study and Completes the NDA Submission for Anaphylm™ (epinephrine) Sublingual Film
Aquestive Therapeutics (NASDAQ: AQST) has announced positive topline pharmacokinetic (PK) results from its pediatric study of Anaphylm™, an epinephrine sublingual film for severe allergic reactions. The study, involving 32 patients aged 7-17 weighing over 30kg, demonstrated consistent PK profiles with previous adult studies, with no serious adverse events reported.
The company has completed and submitted its New Drug Application (NDA) to the FDA, with expected acceptance in Q2 2025. If approved, Anaphylm™ would become the first-ever sublingual film treatment for anaphylaxis, with a planned commercial launch in Q1 2026. The pediatric study results support potential label inclusion for this age group.
Aquestive Therapeutics (NASDAQ: AQST) ha annunciato risultati positivi preliminari di farmacocinetica (PK) dal suo studio pediatrico su Anaphylm™, un film sublinguale di epinefrina per reazioni allergiche gravi. Lo studio, che ha coinvolto 32 pazienti di età compresa tra 7 e 17 anni con un peso superiore a 30 kg, ha dimostrato profili PK coerenti con studi precedenti sugli adulti, senza eventi avversi gravi riportati.
L'azienda ha completato e presentato la sua Domanda di Nuovo Farmaco (NDA) alla FDA, con accettazione prevista nel secondo trimestre del 2025. Se approvato, Anaphylm™ diventerebbe il primo trattamento mai realizzato in forma di film sublinguale per l'anafilassi, con un lancio commerciale pianificato nel primo trimestre del 2026. I risultati dello studio pediatrico supportano una potenziale inclusione dell'etichetta per questo gruppo di età.
Aquestive Therapeutics (NASDAQ: AQST) ha anunciado resultados farmacocinéticos (PK) positivos de su estudio pediátrico sobre Anaphylm™, un filme sublingual de epinefrina para reacciones alérgicas severas. El estudio, que involucró a 32 pacientes de entre 7 y 17 años con un peso superior a 30 kg, demostró perfiles PK consistentes con estudios anteriores en adultos, sin eventos adversos graves reportados.
La empresa ha completado y presentado su Solicitud de Nuevo Medicamento (NDA) a la FDA, con una aceptación esperada en el segundo trimestre de 2025. Si se aprueba, Anaphylm™ se convertiría en el primer tratamiento en forma de filme sublingual para la anafilaxia, con un lanzamiento comercial planeado para el primer trimestre de 2026. Los resultados del estudio pediátrico respaldan una posible inclusión en la etiqueta para este grupo de edad.
Aquestive Therapeutics (NASDAQ: AQST)는 심각한 알레르기 반응을 위한 에피네프린 설하 필름인 Anaphylm™의 소아 연구에서 긍정적인 약리학적 결과를 발표했습니다. 이 연구는 30kg 이상의 체중을 가진 7세에서 17세 사이의 32명의 환자를 포함했으며, 이전의 성인 연구와 일관된 약리학적 프로파일을 보여주었고, 심각한 부작용은 보고되지 않았습니다.
회사는 FDA에 새로운 약물 신청서(NDA)를 제출 완료하였으며, 2025년 2분기에 수락될 것으로 예상하고 있습니다. 승인이 이루어지면, Anaphylm™은 아나필락시스를 위한 최초의 설하 필름 치료제가 되며, 2026년 1분기에 상업 출시가 계획되어 있습니다. 소아 연구 결과는 이 연령대에 대한 라벨 포함 가능성을 뒷받침합니다.
Aquestive Therapeutics (NASDAQ: AQST) a annoncé des résultats pharmacocinétiques (PK) préliminaires positifs de son étude pédiatrique sur Anaphylm™, un film sublingual d'épinéphrine pour les réactions allergiques sévères. L'étude, impliquant 32 patients âgés de 7 à 17 ans pesant plus de 30 kg, a montré des profils PK cohérents avec des études antérieures chez les adultes, sans événements indésirables graves signalés.
L'entreprise a complété et soumis sa Demande de Nouveau Médicament (NDA) à la FDA, avec une acceptation prévue au deuxième trimestre 2025. Si approuvé, Anaphylm™ deviendrait le premier traitement sous forme de film sublingual pour l'anaphylaxie, avec un lancement commercial prévu au premier trimestre 2026. Les résultats de l'étude pédiatrique soutiennent une éventuelle inclusion sur l'étiquette pour ce groupe d'âge.
Aquestive Therapeutics (NASDAQ: AQST) hat positive vorläufige Ergebnisse zur Pharmakokinetik (PK) aus seiner pädiatrischen Studie zu Anaphylm™, einem sublingualen Epinephrin-Film für schwere allergische Reaktionen, bekannt gegeben. Die Studie umfasste 32 Patienten im Alter von 7 bis 17 Jahren mit einem Gewicht von über 30 kg und zeigte konsistente PK-Profile im Vergleich zu früheren Studien an Erwachsenen, ohne schwerwiegende unerwünschte Ereignisse.
Das Unternehmen hat seinen Antrag auf Zulassung eines neuen Arzneimittels (NDA) bei der FDA abgeschlossen und eingereicht, mit einer erwarteten Annahme im zweiten Quartal 2025. Wenn genehmigt, würde Anaphylm™ die erste sublinguale Filmbehandlung für Anaphylaxie werden, mit einem geplanten kommerziellen Launch im ersten Quartal 2026. Die Ergebnisse der pädiatrischen Studie unterstützen eine mögliche Etikettierung für diese Altersgruppe.
- Successful completion of pediatric clinical trials with positive PK results
- No serious adverse events (SAEs) reported in pediatric trials
- NDA submission completed ahead of potential Q1 2026 commercial launch
- Product could be first-in-class sublingual film treatment for anaphylaxis
- FDA approval pending with potential delays possible
- Commercial launch not expected until Q1 2026
Insights
Aquestive's announcement represents a significant milestone in their Anaphylm development program. The positive pharmacokinetic results in the pediatric population (ages 7-17, >30kg) are particularly valuable as they demonstrate consistency with adult studies - a critical factor for regulatory approval. This consistency suggests the company has successfully optimized their proprietary PharmFilm technology for epinephrine delivery across different age groups.
The completion of their NDA submission positions Anaphylm as a potential first-in-class sublingual film for anaphylaxis treatment, which could disrupt a market dominated by auto-injectors. From a clinical perspective, a needle-free alternative addresses significant unmet needs including needle phobia, portability issues, and potential administration barriers during emergency situations.
The safety profile appears favorable with no serious adverse events reported in the pediatric study - a crucial consideration for this vulnerable population and any emergency treatment. Importantly, the company has aligned their regulatory and commercial timelines appropriately, with potential approval and launch in 2026 if the FDA review proceeds as anticipated.
While this represents completion of their clinical program, Aquestive will now face the challenge of transitioning from development to commercialization. Their ability to effectively differentiate Anaphylm from established auto-injectors and secure favorable reimbursement will ultimately determine commercial success if approved.
The completed NDA submission for Anaphylm represents textbook execution of regulatory strategy. By conducting and including pediatric data in their initial submission, Aquestive is positioning for a potentially broader label from the outset - a strategic advantage that could significantly enhance market access if approved.
The FDA's review timeline indicated (Q2 2025 acceptance, potential Q1 2026 launch) suggests Aquestive anticipates a standard 10-month review cycle. This timeline is reasonable given this is a novel delivery system for an established molecule rather than a New Molecular Entity, which might qualify for expedited pathways.
From a regulatory perspective, the consistent pharmacokinetic profile between pediatric and adult populations strengthens their submission considerably. Demonstration of comparable systemic exposure across age groups is precisely what the FDA seeks when evaluating pediatric extensions.
The absence of serious adverse events in the pediatric study removes a potential regulatory hurdle, though the FDA will thoroughly evaluate the complete safety database. The regulatory path forward appears straightforward, but will require strong CMC (Chemistry, Manufacturing and Controls) data to support the novel delivery system alongside the clinical results reported. Aquestive's experience with their proprietary PharmFilm technology in previously approved products likely strengthens their manufacturing readiness position with regulators.
- Reports positive pharmacokinetic (PK) results from the recently completed pediatric study for Anaphylm™
- Completes submission of its New Drug Application (NDA) for Anaphylm to the FDA; NDA acceptance expected in Q2 2025
WARREN, N.J., April 01, 2025 (GLOBE NEWSWIRE) -- Aquestive Therapeutics, Inc. (NASDAQ: AQST) (“Aquestive” or the “Company”), a pharmaceutical company advancing medicines to bring meaningful improvement to patients' lives through innovative science and delivery technologies, today announced positive topline results from its pediatric study for Anaphylm™ (epinephrine) sublingual film in patients aged seven to seventeen and weighing greater that thirty kilograms with a personal history of allergic reactions. This marks the completion of the Anaphylm clinical program and supports the clinical data needed for the NDA submission. Aquestive has submitted the Anaphylm NDA to the U.S. Food and Drug Administration (FDA) and expects to receive potential acceptance of the NDA during the second quarter of 2025.
"We are extremely pleased with the positive results from our pediatric study, which further validate Anaphylm's potential as the first-ever sublingual film and convenient treatment option for all patients with severe allergic reactions, including anaphylaxis," said Daniel Barber, President and Chief Executive Officer of Aquestive. "These results demonstrate that Anaphylm maintains its consistent PK profile in pediatric patients between the ages of seven and seventeen and weighing greater than thirty kilograms. These data are an important component of the FDA review process and could enable Anaphylm to have a label that includes this pediatric patient population. We continue to prepare for commercial readiness and plan to launch Anaphylm in the first quarter of 2026, if approved by the FDA.”
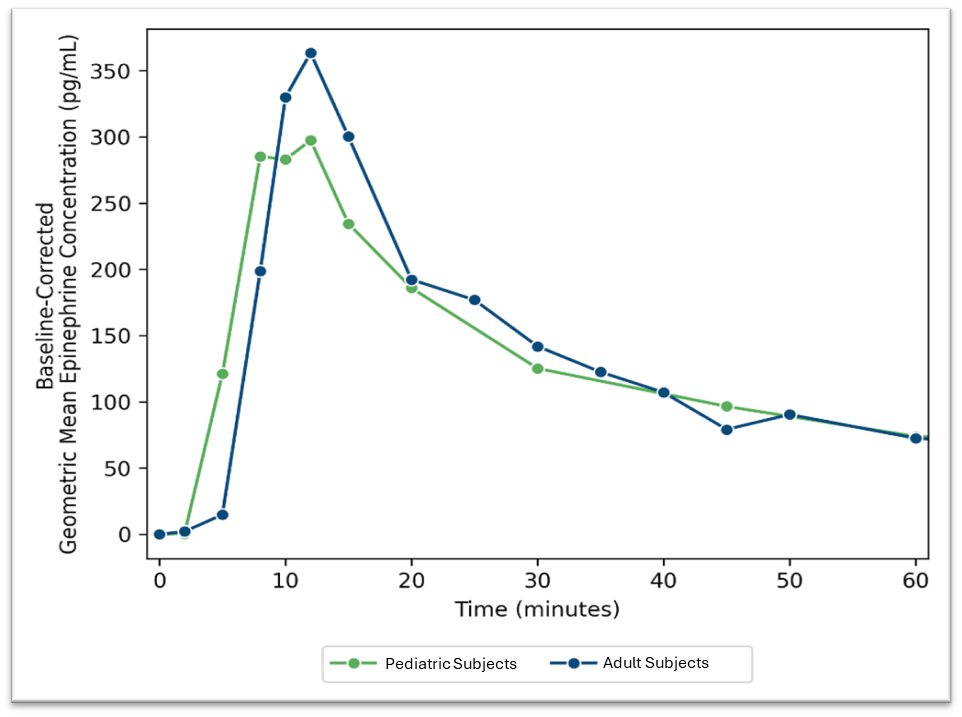
The pediatric study was a multi-site, single treatment study designed to evaluate the PK, pharmacodynamics (PD), safety, and tolerability of Anaphylm. A total of thirty-two patients completed the study. The PK results were consistent with previous adult studies. Importantly, Anaphylm was shown to be safe and well-tolerated with no serious adverse events (SAEs) reported. Figure 1 below compares the pediatric study results to the previously announced pivotal adult study results.
Figure 1: Geometric mean baseline adjusted epinephrine concentration over time after administration of Anaphylm 12mg in pediatric subjects (Study AQ109302) compared to adult subjects (Study AQ109301B)
About Anaphylm™ (epinephrine) Sublingual Film
Anaphylm™ (epinephrine) Sublingual Film is a polymer matrix-based epinephrine prodrug product candidate in development for the treatment of severe allergic reactions, including anaphylaxis. Anaphylm is similar in size to a postage stamp, weighs less than an ounce, and begins to dissolve on contact. No water or swallowing is required for administration. The packaging for Anaphylm is thinner and smaller than an average credit card, can be carried in a pocket, and is designed to withstand weather excursions such as exposure to rain and/or sunlight. The Anaphylm trade name for AQST-109 has been conditionally approved by the FDA. Final approval of the Anaphylm proprietary name is conditioned on FDA approval of the product candidate.
About Aquestive Therapeutics
Aquestive is a pharmaceutical company advancing medicines to bring meaningful improvement to patients' lives through innovative science and delivery technologies. We are developing orally administered products to deliver complex molecules, providing novel alternatives to invasive and inconvenient standard of care therapies. Aquestive has five commercialized products marketed by the Company and its licensees in the U.S. and around the world, and is the exclusive manufacturer of these licensed products. The Company also collaborates with pharmaceutical companies to bring new molecules to market using proprietary, best-in-class technologies, like PharmFilm®, and has proven drug development and commercialization capabilities. Aquestive is advancing a late-stage proprietary product candidate for the treatment of severe allergic reactions, including anaphylaxis, and an earlier stage epinephrine prodrug topical gel for possible various dermatology conditions, including alopecia areata. For more information, visit Aquestive.com and follow us on LinkedIn.
Forward-Looking Statement
Certain statements in this press release include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believe,” “anticipate,” “plan,” “expect,” “estimate,” “intend,” “may,” “will,” or the negative of those terms, and similar expressions, are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the advancement and related timing of our product candidate Anaphylm™ (epinephrine) Sublingual Film through clinical development and approval by the FDA, including the timing and acceptance of the NDA for Anaphylm with the FDA and potential approved label indications, and the following launch of Anaphylm, if approved by the FDA; the results of the Company’s clinical studies for Anaphylm and the ability of such results to support submission of the NDA for approval of Anaphylm to the FDA; Anaphylm's potential as a non-device, non-invasive based treatment option for severe allergic reactions, including anaphylaxis, if Anaphylm is approved by the FDA; the advancement, growth and related timing of our Adrenaverse™ pipeline of epinephrine prodrug product candidates, including AQST-108 (epinephrine) Topical Gel through clinical development and FDA regulatory approval process, including design and timing of clinical studies including those necessary to support the targeted indication of alopecia areata for AQST-108; the potential benefits our products and product candidates could bring to patients; and business strategies, market opportunities, and other statements that are not historical facts.
These forward-looking statements are based on our current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Such risks and uncertainties include, but are not limited to, risks associated with our development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials and plans, including those relating to Anaphylm (including for pediatric patients), AQST-108, and our other product candidates; risk of delays in advancement of the regulatory approval process through the FDA of our product candidates, including the filing of the respective NDAs, for Anaphylm, AQST-108and other product candidates, or failure to receive FDA approval at all of any of these product candidates; risk of the Company’s ability to generate sufficient clinical data for approval of our product candidates, including with respect to our PK/PD comparability submission for FDA approval of Anaphylm; risks associated with our ability to address the FDA’s comments on our future clinical trials and other concerns identified in the FDA Type C meeting minutes for Anaphylm, including the risk that the FDA may require additional clinical studies for approval of Anaphylm; risks associated with the success of any competing products, including generics; risks and uncertainties inherent in commercializing a new product (including technology risks, financial risks, market risks and implementation risks and regulatory limitations); risk of development of a sales and marketing capability for commercialization of our product candidates, including Anaphylm and AQST-108; risks associated with the potential impact on the value of the Company of the sale or outlicensing of our product and product candidates, including Anaphylm and other product candidates; risk of insufficient capital and cash resources, including insufficient access to available debt and equity financing, including under our ATM facility, and revenues from operations, to satisfy all of our short-term and longer-term liquidity and cash requirements and other cash needs, at the times and in the amounts needed, including to fund commercialization activities relating to future clinical development and commercial activities for our product candidates, including Anaphylm and AQST-108, should these product candidates be approved by the FDA; risk that our manufacturing capabilities will be insufficient to support demand for our licensed products in the U.S. and abroad; risk of eroding market share for Suboxone® as a sunsetting product, which accounts for a substantial part of our current operating revenue; risk of default of our debt instruments; risks related to the outsourcing of certain sales, marketing and other operational and staff functions to third parties; risk of the rate and degree of market acceptance in the U.S. and abroad of Anaphylm, AQST-108 and our other product candidates, should these product candidates be approved by the FDA, and for our licensed products in the U.S. and abroad; risk associated with the size and growth of our product markets; risk associated with our compliance with all FDA and other governmental and customer requirements for our manufacturing facilities; risks associated with intellectual property rights and infringement claims relating to our products; risk that our patent applications for our product candidates, including for Anaphylm and AQST-108, will not be timely issued, or issued at all, by the U.S. Patent and Trademark Office; risk of unexpected patent developments; risk of legislation and regulatory actions and changes in laws or regulations affecting our business including relating to our products and product candidates and product pricing, reimbursement or access therefor; risk of loss of significant customers; risks related to claims and legal proceedings against us including patent infringement, securities, business torts, investigative, product safety or efficacy and antitrust litigation matters; risk of product recalls and withdrawals; risks related to any disruptions in our information technology networks and systems, including the impact of cybersecurity attacks; risk of increased cybersecurity attacks and data accessibility disruptions due to remote working arrangements; risk of adverse developments affecting the financial services industry; risks related to inflation and changing interest rates; risks related to the impact of other pandemic diseases, such as COVID-19, on our business; risks and uncertainties related to general economic, political (including the Ukraine and Israel wars and other acts of war and terrorism), business, industry, regulatory, financial and market conditions and other unusual items; risks related to uncertainty about presidential administration initiatives and their impact on our business; and other uncertainties affecting us including those described in the "Risk Factors" section and in other sections included in the Company’s 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the U.S. Securities and Exchange Commission. Given those uncertainties, you should not place undue reliance on these forward-looking statements, which speak only as of the date made. All subsequent forward-looking statements attributable to the Company or any person acting on its behalf are expressly qualified in their entirety by this cautionary statement. The Company assumes no obligation to update forward-looking statements or outlook or guidance after the date of this press release whether as a result of new information, future events or otherwise, except as may be required by applicable law.
PharmFilm® and the Aquestive logo are registered trademarks of Aquestive Therapeutics, Inc.
Investor Inquiries
Astr Partners
Brian Korb
brian.korb@asterpartners.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f8452a4a-c453-4def-8656-7e571a206bf7








