RENUVION® DERMAL HANDPIECE FOR WRINKLE-REDUCTION PROCEDURES CLEARED BY FDA
Apyx Medical Corporation (NASDAQ: APYX) announced FDA clearance for its Renuvion Dermal handpiece, allowing for wrinkle reduction procedures in patients with Fitzpatrick skin types I, II, or III. The new treatment, branded as Facial Renewal, offers a nonsurgical method for skin rejuvenation, providing patients with natural-looking results without invasive procedures. According to Dr. Tabasum Mir, the treatment addresses a significant need in cosmetic options. Apyx's President, Charlie Goodwin, highlighted this advancement as a milestone in cosmetic treatments.
- None.
- None.
Insights
Analyzing...
Revolutionary Apyx Medical Device Used by Top Physicians in the US Now Available to Patients for Nonsurgical Facial Renewal
CLEARWATER, Fla., June 28, 2022 /PRNewswire/ -- Apyx Medical Corporation (NASDAQ: APYX), the manufacturer of Renuvion®, a proprietary helium plasma and radiofrequency technology, previously announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration ("FDA") for the use of the Renuvion Dermal handpiece for specific dermatological procedures for the treatment of moderate to severe wrinkles and rhytides, limited to patients with Fitzpatrick skin types I, II or III.
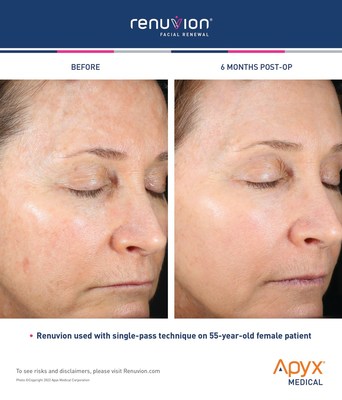
Branded as Facial Renewal, this treatment for wrinkle-reduction provides patients with an effective and nonsurgical option for smoothing and contracting the skin in one procedure. Facial Renewal enables dermatologists, plastic surgeons and cosmetic physicians to achieve transformative results, without the patient needing invasive surgery or risking looking unnatural. Facial Renewal with Renuvion is the first major advancement in decades of skin treatments for the face, providing patients with dramatic and natural-looking results.
"Renuvion for facial procedures is groundbreaking. It's a treatment that fulfills a big unmet need in cosmetic procedures," said New York oculoplastic surgeon Tabasum Mir, MD. "I have been using the Renuvion technology on patients in my practice and I've seen the transformative results firsthand. With the new clearance for Facial Renewal, this opens a whole new treatment option for patients to achieve the youthful appearance they're seeking."
"We are very pleased to receive FDA clearance with a specific clinical indication that enables Apyx Medical to market and sell our Renuvion Technology to physicians and patients for use in dermal resurfacing procedures," said Charlie Goodwin, President and Chief Executive Officer. "Facial Renewal with Renuvion offers patients a refreshed and more youthful look without having the unnatural 'pulled' appearance often associated with a facelift."
For more information visit Renuvion.com.
Apyx Medical Corporation is an advanced energy technology company with a passion for elevating people's lives through innovative products in the cosmetic and surgical markets. Known for its innovative Helium Plasma Technology, Apyx is solely focused on bringing transformative solutions to the physicians and patients it serves. The company's Helium Plasma Technology is marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion® offers surgeons and physicians a unique ability to provide controlled heat to the tissue to achieve their desired results. The J-Plasma® system allows surgeons to operate with a high level of precision while minimizing unintended tissue trauma. The Company also leverages its deep expertise and decades of experience in unique waveforms through original equipment manufacturing (OEM) agreements with other medical device manufacturers. For further information about the company and its products, please refer to the Apyx Medical Corporation website at www.ApyxMedical.com.
Certain matters discussed in this release and oral statements made from time to time by representatives of the Company may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Although the Company believes that the expectations reflected in such forward-looking statements are based upon reasonable assumptions, it can give no assurance that its expectations will be achieved.
All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including but not limited to, any statements regarding the potential impact of the COVID-19 pandemic and the actions by governments, businesses and individuals in response to the situation; projections of net revenue, margins, expenses, net earnings, net earnings per share, or other financial items; projections or assumptions concerning the possible receipt by the Company of any regulatory approvals from any government agency or instrumentality including but not limited to the U.S. Food and Drug Administration, supply chain disruptions, component shortages, manufacturing disruptions or logistics challenges; or macroeconomic or geopolitical matters and the impact of those matters on the Company's financial performance.
Forward-looking statements and information are subject to certain risks, trends and uncertainties that could cause actual results to differ materially from those projected. Many of these factors are beyond the Company's ability to control or predict. Important factors that may cause the Company's actual results to differ materially and that could impact the Company and the statements contained in this release include but are not limited to risks, uncertainties and assumptions relating to the regulatory environment in which the Company is subject to, including the Company's ability to gain requisite approvals for its products from the U.S. Food and Drug Administration and other governmental and regulatory bodies, both domestically and internationally; the impact of the recent FDA Safety Communication on our business and operations; factors relating to the effects of the COVID-19 pandemic; sudden or extreme volatility in commodity prices and availability, including supply chain disruptions; changes in general economic, business or demographic conditions or trends; changes in and effects of the geopolitical environment; liabilities and costs which the Company may incur from pending or threatened litigations, claims, disputes or investigations; and other risks that are described in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2021 and the Company's other filings with the Securities and Exchange Commission. For forward-looking statements in this release, the Company claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. The Company assumes no obligation to update or supplement any forward-looking statements whether as a result of new information, future events or otherwise.
Media Contact:
Jane Sparango
Coterie Media (for Renuvion)
jane@coteriemedia.com
310-339-1214
Investor Relations Contact:
ICR Westwicke on behalf of Apyx Medical Corporation
Mike Piccinino, CFA
investor.relations@apyxmedical.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/renuvion-dermal-handpiece-for-wrinkle-reduction-procedures-cleared-by-fda-301576519.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/renuvion-dermal-handpiece-for-wrinkle-reduction-procedures-cleared-by-fda-301576519.html
SOURCE Renuvion