Autonomix Announces 83% Reduction of Pain at 4-6 Week Follow-Up from Lead-In Patients in Ongoing Human Clinical Trial in Pancreatic Cancer Pain Patients
Rhea-AI Summary
Autonomix Medical (NASDAQ: AMIX) has announced promising 4-6 week preliminary results from its ongoing proof-of-concept human clinical trial for pancreatic cancer pain treatment. The trial, evaluating the safety and effectiveness of transvascular energy ablation of problematic nerves, showed significant pain reduction in responders:
- 60% of patients responded with a mean 83% reduction in pain (VAS scale: 8.0 to 1.33)
- 66% of responders reported a VAS pain score of 0 or 1
- 100% of responders experienced clinically meaningful pain relief
- Pain relief was observed as early as 1-day post-procedure
The company's proprietary technology uses a catheter-based microchip sensing array antenna to detect neural signals with high sensitivity, followed by precision-guided RF ablation. The trial is on track to complete enrollment by year-end 2024.
Positive
- 60% of patients responded with an 83% reduction in pain (VAS scale: 8.0 to 1.33) at 4-6 weeks post-procedure
- 66% of responders reported a VAS pain score of 0 or 1 with a greater than 7-point reduction in pain score
- 100% of responders experienced clinically meaningful pain relief at 4-6 weeks post-procedure
- Pain relief was observed as early as 1-day post-procedure for responders
- Trial remains on track to complete enrollment by year-end 2024
Negative
- 40% of patients (2 out of 5) showed no improvement in pain scores
- One subject succumbed to their disease prior to the 4-6 week follow-up appointment
News Market Reaction – AMIX
On the day this news was published, AMIX gained 9.09%, reflecting a notable positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Responders experienced a pain reduction from a pre-procedure pain score of 8 to a pain score of 1.33 at 4-6 weeks post-procedure
Trial remains on track to complete enrollment by year-end 2024
THE WOODLANDS, TX, Sept. 09, 2024 (GLOBE NEWSWIRE) -- Autonomix Medical, Inc. (NASDAQ: AMIX) (“Autonomix” or the “Company”), a medical device company focused on advancing precision nerve-targeted treatments, today announced 4-6 week preliminary positive results from the first five “lead-in” patients in the Company’s ongoing proof-of-concept human clinical trial (the “Trial”) evaluating the safety and effectiveness of delivering transvascular energy to ablate relevant problematic nerves and mitigate pain in patients with pancreatic cancer pain.
“We are encouraged by the significant, sustained pain reduction demonstrated from the lead-in patients in our ongoing first-in-human trial. These are very sick individuals, who up until this point primarily relied on opioids to help mitigate their pain, which our baseline data shows were not effective. Following our treatment, all the responders had a clinically meaningful mean reduction in pain from 8 to 1.33 on the VAS pain scale at 4-6 weeks post-procedure,” commented Brad Hauser, Chief Executive Officer of Autonomix. “While these data are preliminary, we are excited about our precision nerve-targeted technology and the potential it has to revolutionize electrophysiology therapies.”
Summary of Topline Results 4-6 Weeks Post-Procedure
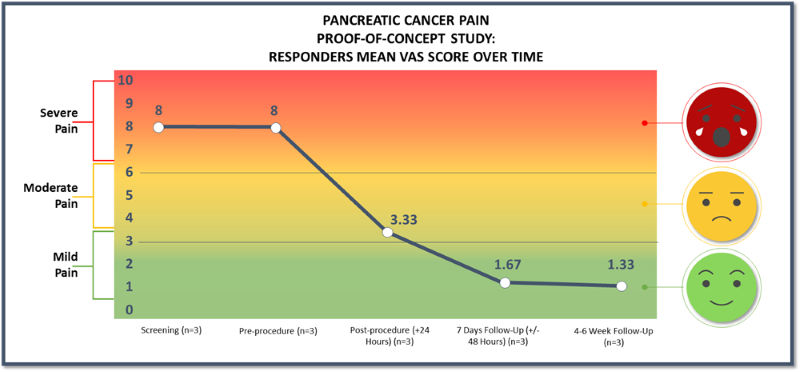
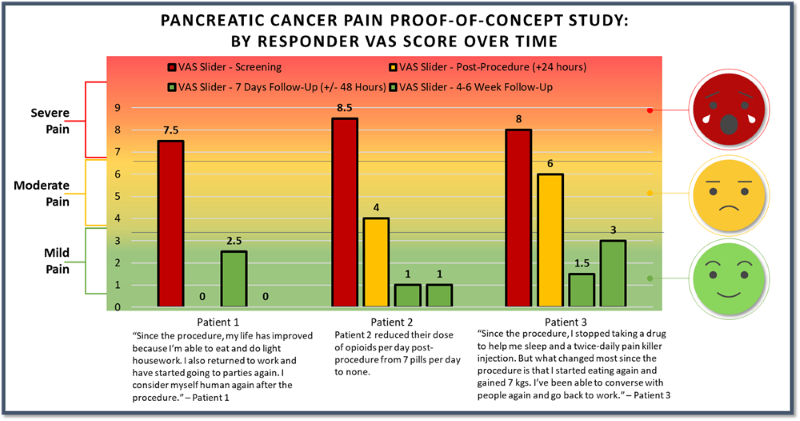
- As previously announced, three patients were treated with femoral access and two were treated with brachial access. All patients treated with femoral access positively responded to treatment and patients treated with brachial access showed no improvement in their pain scores. The results presented in the charts above are for the three patients in the responder group.
60% of patients responded with a mean of 6.67, or83% , reduction of pain on the VAS pain scale (from baseline of 8.0 to 1.33) at 4-6 weeks post-procedure.66% of responders reported a VAS pain score of 0 or 1 with a greater than 7-point reduction in pain score at 4-6 weeks post-procedure.100% of our responder group had clinically meaningful pain relief at 4-6 weeks post-procedure.- Pain relief was experienced as quick as 1-day post-procedure for the three patients in the responder group.
- Reduction of pain score occurred simultaneously as the subject’s underlying disease (pancreatic cancer tumor) continued to grow. This is beneficial for patients who are considered end of life given their advanced stage of disease.
When evaluating the total treated population, including non-responders, the mean reduction in the VAS pain score was a 5.125 point reduction, or
As previously announced, Autonomix has amended the trial protocol to include the gathering of additional information on tumor encroachment on the vessels, as well as other key bio-measurements that may correlate with effective nerve ablation. Additionally, the Company has further defined severe pain for inclusion criteria as a 7 or above on the VAS scale as indicated by the patient rather than physician determination. A total of twenty (20) additional patients will be enrolled in the trial that will be formally included in the trial data results and analysis of trial objectives. Suitability is determined by the primary oncologist caring for the patients with the treating Principal Investigator confirming eligibility for the trial. Autonomix commenced patient screening under the amended protocol in May 2024 and remains on track to complete enrollment in the Trial by year-end.
The Company’s first-in-class technology platform utilizes a catheter-based microchip sensing array antenna that has the ability to detect and differentiate neural signals with up to 3,000 times greater sensitivity than currently available technologies. Once target nerves are identified, Autonomix uses its proprietary radio frequency (RF) ablation technology to kill targeted nerves, enabling a precision guided sense, treat and verify approach to addressing a number of disease categories from chronic pain management to hypertension and cardiology. Current approaches, primarily relying on opioids or invasive ethanol injections, can provide only limited relief and may lead to risky side effects. For more information about the Company’s technology, please visit autonomix.com.
About the Trial
The goal of the Trial is to assess pain reduction via radiofrequency (RF) ablation. The Company’s catheter-based microchip sensing array used to detect and differentiate neural signaling was not used in this trial and will be evaluated in future studies.
The first five patients were enrolled and treated according to protocol in the beginning of the trial to familiarize the Principal Investigator (PI) with the procedure and will not be included in the analysis of the trial objectives. These first five “lead-in” patients successfully completed the procedure per protocol with no immediate procedural-related complications or significant adverse events.
The primary objective of the Trial is to assess the success rate of ablating relevant nerves to mitigate pain in patients with pancreatic cancer pain utilizing RF ablation in a transvascular approach to the nerves in the region. Secondary objectives include assessing the incidence of device- and procedure-related adverse events up to 4-6 weeks post-procedure; estimating the change in pain levels from pre- to post-procedure; and estimating the change in quality of life from pre- to post-procedure. All patients who have had a successful procedure will be evaluated at 7 days, 4–6 weeks, and at 3 months post-procedure. All patients entered the study with severe abdominal pain from unresectable pancreatic cancer and a life expectancy of 3 months or less. Following the successful completion of the procedure, two patients have since succumbed to their disease. Both events were expected outcomes and not related to the trial procedure.
About Autonomix Medical, Inc.
Autonomix is a medical device company focused on advancing innovative technologies to revolutionize how diseases involving the nervous system are diagnosed and treated. The Company’s first-in-class technology platform includes a catheter-based microchip sensing array that has the ability to detect and differentiate neural signals with approximately 3,000 times greater sensitivity than currently available technologies. We believe this will enable, for the first time ever, transvascular diagnosis and treatment of diseases involving the peripheral nervous system virtually anywhere in the body.
We are initially developing this technology for the treatment of pain, with initial trials focused on pancreatic cancer, a condition that causes debilitating pain and is without a reliable solution. Our technology constitutes a platform to address dozens of indications, including cardiology, hypertension and chronic pain management, across a wide disease spectrum. Our technology is investigational and has not yet been cleared for marketing in the United States.
For more information, visit autonomix.com and connect with the Company on X, LinkedIn, Instagram and Facebook.
Forward Looking Statements
Some of the statements in this release are “forward-looking statements,” which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the potential of the technology to treat pain associated with pancreatic cancer, to successfully enroll patients within the specific timeframe, and to complete its clinical study in pancreatic cancer pain. Such forward-looking statements can be identified by the use of words such as “should,” “might,” “may,” “intends,” “anticipates,” “believes,” “estimates,” “projects,” “forecasts,” “expects,” “plans,” and “proposes.”
Although Autonomix believes that the expectations reflected in these forward-looking statements are based on reasonable assumptions, there are a number of risks and uncertainties that could cause actual results to differ materially from such forward-looking statements. You are urged to carefully review and consider any cautionary statements and other disclosures, including the statements made under the heading “Risk Factors” and elsewhere in the Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC) on May 31, 2024. Forward-looking statements speak only as of the date of the document in which they are contained and Autonomix does not undertake any duty to update any forward-looking statements except as may be required by law.
Investor and Media Contact
JTC Team, LLC
Jenene Thomas
833-475-8247
autonomix@jtcir.com
Attachments








