Amgen Releases 8th Edition of Biosimilar Trends Report
On Sept. 22, 2021, Amgen released its 8th edition of the Biosimilar Trends Report, highlighting the U.S. biosimilars marketplace across inflammation, oncology, and nephrology. The report indicates that biosimilars have saved the U.S. healthcare system $9.8 billion over five years and could reduce patient out-of-pocket spending by $238 million. It notes that biosimilars launch with prices 15% to 37% lower than reference products, capturing an average market share of 65% in therapeutic areas. The report underscores the importance of competition and education for the sustainable growth of biosimilars.
- Biosimilars have saved the U.S. healthcare system $9.8 billion over the past five years.
- Potential to reduce patient out-of-pocket spending by $238 million in nine biologic drug classes.
- Biosimilars launch at prices 15% to 37% lower than reference products, increasing competition.
- Average market share of biosimilars in newly launched therapeutic areas is 65%.
- None.
Insights
Analyzing...
THOUSAND OAKS, Calif., Sept. 22, 2021 /PRNewswire/ -- Amgen (NASDAQ: AMGN) today released the 8th edition of the Biosimilar Trends Report, which examines the current state of the U.S. biosimilars marketplace across inflammation, oncology and nephrology categories, while a new feature considers how advancements in biosimilars can support the long-term success and sustainability of the U.S. healthcare system. To access the full report, visit: www.amgenbiosimilars.com/commitment/trends-report.
To view the multimedia assets associated with this release, please click: https://www.multivu.com/players/English/8812854-amgen-8th-edition-biosimilar-trends-report/
The Report shows that competition created by biosimilars has saved the U.S. healthcare system
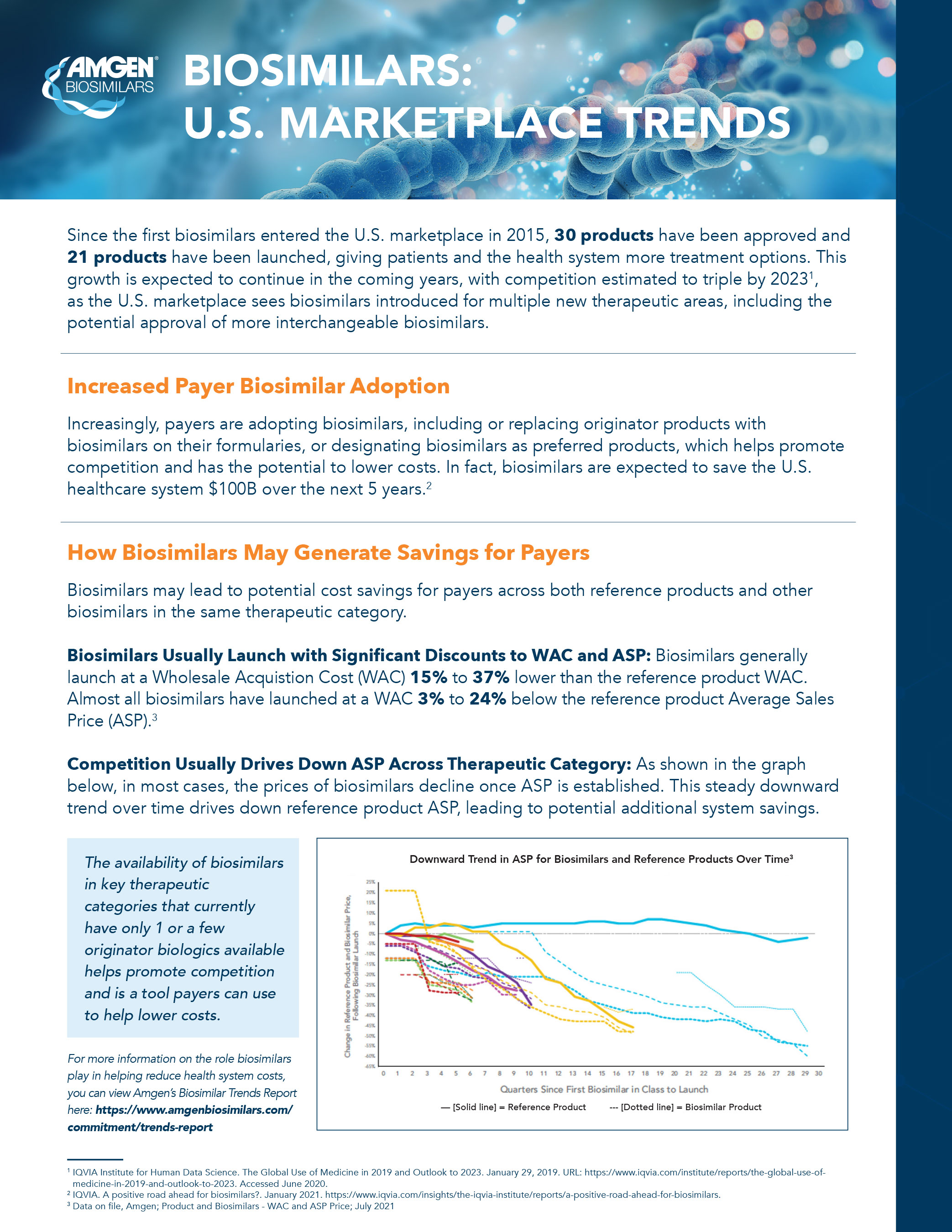
- Biosimilars contribute to competition that drives down healthcare costs by providing significant wholesale acquisition cost (WAC) and average sales price (ASP) discounts at launch, resulting in additional savings over time.
- Biosimilars are launching at a price that is generally
15% to37% lower than the reference product.ii Furthermore, the ASP is declining for both reference products and biosimilars over time, while the rate of biosimilar uptake is generally increasing over time.iii,iv - Biosimilars have gained substantial share in the majority of therapeutic areas where they have been introduced.v For therapeutic areas with biosimilars launched in the last two years, the average share was
65% .vi
This year's Report features a new section, Future State of the Marketplace, which outlines how biosimilars can continue to offer more affordable treatment options, drive cost savings through increased competition between biosimilars and with reference biologics, and promote a more resilient U.S. healthcare marketplace. The U.S. marketplace is poised to see further growth in biosimilars approved to date and welcome many new biosimilars in the years to come.
"As part of Amgen's commitment to staying at the forefront of biosimilar education, Amgen is pleased to announce the launch of the 2021 Biosimilar Trends Report," said Jennifer Norton, vice president, Head of U.S. Value and Access at Amgen. "The increasing availability and adoption of biosimilars means these treatments are delivering on the fundamental promise of reducing healthcare costs for payers, employers, and patients in the United States."
The 2021 Biosimilar Trends Report also outlines the four key elements that Amgen believes are necessary for sustaining biosimilars' growth:
- Implementing scientifically appropriate regulatory standards for the approval, manufacture, and uninterrupted availability of safe and effective biological products, including biosimilars;
- Maintaining a marketplace that encourages competition on a level playing field to achieve meaningful savings and long-term stability;
- Providing scientifically accurate educational outreach to drive confidence with healthcare providers, patients, payers, and employers; and
- Ensuring a foundation of strong intellectual property to encourage innovation and investment.
"The U.S. marketplace with biosimilars is well established and accelerating across key therapeutic areas, creating cost savings, and additional treatment options for physicians and patients," said Chad Pettit, executive director, Marketing, Global Biosimilars Commercial Lead at Amgen, adding, "By continuing to advance science-based policies that support competition and enhance confidence from patients, physicians, and other stakeholders, the U.S. can help promote a robust and resilient healthcare system needed for the long term."
About Amgen Biosimilars
Amgen is committed to building upon Amgen's experience in the development and manufacturing of innovative human therapeutics to expand Amgen's reach to patients with serious illnesses. Biosimilars help to maintain Amgen's commitment to connect patients with vital medicines, and Amgen is well positioned to leverage its nearly four decades of experience in biotechnology to create high-quality biosimilars and reliably supply them to patients worldwide.
For more information, visit www.amgenbiosimilars.com and follow us on www.twitter.com/amgenbiosim.
About Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world's leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
CONTACT: Amgen, Thousand Oaks
Megan Fox, 805-447-1423 (media)
Trish Rowland, 805-447-5631(media)
Arvind Sood, 805-447-1060 (investors)
i Data on file, Amgen; Biosimilars Spend Analysis; July 2021.
ii Data on file, Amgen; Product and Biosimilars - WAC and ASP Price; July 2021.
iii Data on file, Amgen; Product and Biosimilars - WAC and ASP Price; July 2021
iv Data on file, Amgen; Biosimilars Market Share Trends; July 2021
v Data on file, Amgen; Biosimilars Market Share Trends; July 2021
vi Data on file, Amgen; Biosimilars Market Share Trends; July 2021

![]() View original content:https://www.prnewswire.com/news-releases/amgen-releases-8th-edition-of-biosimilar-trends-report-301382173.html
View original content:https://www.prnewswire.com/news-releases/amgen-releases-8th-edition-of-biosimilar-trends-report-301382173.html
SOURCE Amgen







