Alpine Immune Sciences Shares Updated Clinical Data from Povetacicept in IgA Nephropathy
-- Povetacicept administered subcutaneously once every four weeks continues to be well tolerated in IgA nephropathy, with UPCR reductions of greater than
-- Company reports successful end of phase 2 meeting with FDA, supporting advancement to a registrational, placebo-controlled phase 3 study of povetacicept in IgA nephropathy, targeted in 2H 2024 --
-- Company will host an investor call and webcast today at 5:15 pm ET --
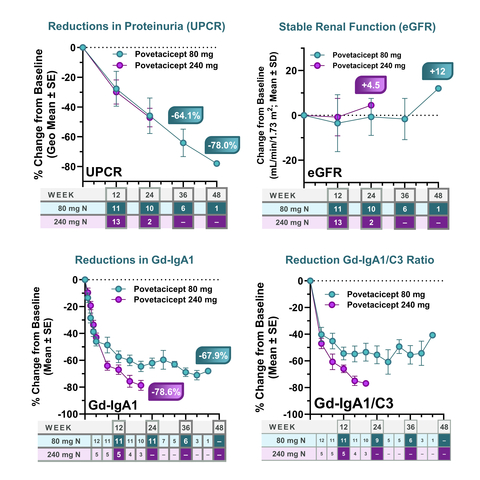
Figure 1. Povetacicept is Associated with Clinically Meaningful UPCR Reductions, Stable eGFR in IgA Nephropathy, and Reductions in Gd-IgA1 and Gd-IgA1/C3 Ratio (Graphic: Business Wire)
Povetacicept is a potent dual antagonist of the BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) cytokines, which play key roles in pathogenesis of multiple autoimmune diseases via their roles in the activation, differentiation and/or survival of B cells, particularly antibody-secreting cells, as well as T cells and innate immune cells. RUBY-3 is a multiple ascending dose, multi-cohort, open label, phase 1b/2a study of povetacicept in autoimmune glomerulonephritis, including IgA nephropathy, where povetacicept is administered subcutaneously (SC) once every four weeks.
Key Highlights Include:
-
As of March 01, 2024, 41 patients with IgAN had received povetacicept 80 or 240 mg subcutaneously every 4 weeks. Treatment with povetacicept 80 mg SC Q4W has been associated with a clinically meaningful improvement in proteinuria, with a
64.1% reduction from baseline in urine protein to creatinine ratio (UPCR; n=6) at 36 weeks, associated with stable renal function as assessed by estimated glomerular filtration rate (eGFR). At this same time, 4/6 (67% ) had achieved remission, as defined as UPCR < 0.5 g/g, ≥50% reduction in UPCR from baseline, and stable renal function (≤25% reduction in eGFR from baseline). -
Resolution of hematuria, as defined as negative or trace/small hematuria in patients with non-negative/trace hematuria at baseline, was achieved in all (
100% ) patients with data available at 36 or more weeks (N=4). - Treatment with povetacicept 240 mg SC Q4W has been associated with similar improvements in proteinuria, stable renal function, and remission, based on initial data through 12 (13 patients) and 24 (2 patients) weeks.
-
Treatment with both doses was associated with significant reductions in the key disease-related biomarker Gd-IgA1, with the 80 mg dose achieving a
68.9% reduction at 40 weeks, and the 240 mg dose achieving a78.6% reduction at 20 weeks. Both doses achieved reductions in IgA/C3 and Gd-IgA1/C3 ratios, which are additional severity and prognostic biomarkers in IgAN. - Both doses have been well tolerated in IgAN, with no instances of IgG < 3 g/L and no severe infections.
“These updated findings for povetacicept are highly promising and continue to support the potential for the program as an important new therapy in IgA nephropathy,” noted Jonathan Barratt, M.D., the Mayer Professor of Renal Medicine and Honorary Consultant Nephrologist and IgA Nephropathy Rare Disease Group Lead, University of Leicester. “The marked and clinically meaningful improvement in UPCR and stable eGFR observed with a monthly dosing regimen at 36 weeks are particularly impressive, as are the deep reductions in Gd-IgA1, a key disease-related biomarker, and resolution of hematuria in patients. The lack of serious adverse events with longer duration of treatment and higher dose is also notable and, together with povetacicept’s disease modifying potential and substantial patient experience, strongly supports the rapid advancement of povetacicept into a pivotal study for IgA nephropathy.”
“Despite advancements in IgA nephropathy, there remains an urgent need for safe and convenient therapies that effectively treat the underlying cause of the disease,” said James Tumlin, M.D., Professor of Medicine at Emory University School of Medicine, Founder and CEO of NephroNet Clinical Trials Consortium. “Dual APRIL/BAFF inhibition is a potent inhibitor of Galactose Deficient IgA1 (Gd-IgA1) production which is closely linked to the pathogenesis and progression of IgA nephropathy. Monthly administration of povetacicept has been shown to be well-tolerated at both the 80mg and 240 mg doses with clinical responses including Gd-IgA1, urine protein levels and eGFR stabilization within 3 months. These data strongly support the inhibition of APRIL/BAFF pathways by povetacicept and its efficacy in the treatment of IgAN as well as the need for further clinical development. If approved, povetacicept could be used a front-line disease modifying treatment in IgAN.”
“These data indeed continue to further our enthusiasm for the potential of povetacicept in IgAN and other autoimmune diseases,” remarked Stanford Peng, MD PhD, President and Head of Research and Development at Alpine. “We are pleased to have completed our successful end of phase 2 meeting with FDA, enabling advancement to a registrational trial in IgAN (RAINIER) later this year. In addition, we plan to continue to explore povetacicept’s potential in multiple other autoimmune diseases via the ongoing RUBY-3 and RUBY-4 studies, and via the initiation of a phase 2 study (DENALI) in systemic lupus erythematosus later this year.”
World Congress of Nephrology Late-Breaking Poster
Date/Time: Monday, April 15, 2024, 5:45 pm local time (GMT-3) /4:45 pm ET/1:45 pm PT
Poster Title: Updated Results from the RUBY-3 study of Povetacicept, an Enhanced Dual BAFF/APRIL Antagonist in IgA Nephropathy
Poster Number: MON-304
Session Name: Poster Session 3 (Late Breaking)
Location: Exhibition Hall and Main Foyer,
Presenter: James Tumlin, M.D., Professor of Medicine at Emory University School of Medicine, Founder and CEO of NephroNet Clinical Trials Consortium
Alpine will host an investor call and webcast to discuss the data update as well as provide a corporate update.
The link to the webcast is available in the investor relations section of the Company’s website at https://ir.alpineimmunesciences.com/events and a replay will be available on the Company's website for 90 days following the live event.
About Povetacicept (ALPN-303)
Povetacicept (ALPN-303) is a dual antagonist of the BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) cytokines, which play key roles in pathogenesis of multiple autoimmune diseases via their roles in the activation, differentiation and/or survival of B cells, particularly antibody-secreting cells, as well as T cells and innate immune cells. Based upon an engineered TACI (transmembrane activator and CAML interactor) domain, povetacicept has exhibited greater potency in preclinical studies versus other inhibitors of BAFF and/or APRIL alone and B cell depletion and has demonstrated initial activity in patients with IgA nephropathy. Povetacicept is in development for multiple autoimmune diseases, including IgA nephropathy and other autoimmune kidney diseases, systemic lupus erythematosus, and autoimmune cytopenias.
About RUBY-3
RUBY-3 (NCT05732402) is a multiple ascending dose, multi-cohort, open label, phase 1b/2 study of povetacicept in autoimmune glomerulonephritis, including IgA nephropathy, primary membranous nephropathy, lupus nephritis, and renal ANCA-associated vasculitis, where povetacicept is being administered subcutaneously for up to 104 weeks. Key endpoints include proteinuria, eGFR, renal response, and disease-related autoantibodies.
About RUBY-4
RUBY-4 (NCT05757570) is a multi-cohort, open label study of povetacicept in autoimmune cytopenias, including immune thrombocytopenia, autoimmune hemolytic anemia, and cold agglutinin disease, where povetacicept is being administered subcutaneously for up to 48 weeks. Key endpoints include respective blood cell counts, durable responses, as well as disease-related autoantibodies.
About RAINIER
RAINIER is an upcoming, randomized, double-blind, placebo-controlled, pivotal phase 3 study to evaluate the safety and efficacy of povetacicept in patients with IgA nephropathy at risk of progression to kidney failure.
About DENALI
DENALI is an upcoming, randomized, double-blind, placebo-controlled phase 2 study to evaluate the safety and efficacy of povetacicept in patients with active systemic lupus erythematosus.
About Alpine Immune Sciences
Alpine Immune Sciences is committed to leading a new wave of immune therapeutics. With world-class research and development capabilities, a highly productive scientific platform, and a proven management team, Alpine is seeking to create first- or best-in-class multifunctional immunotherapies via unique protein engineering technologies to improve patients’ lives. Alpine has entered into strategic collaborations with leading global biopharmaceutical companies and has a diverse pipeline of clinical and preclinical candidates in development. For more information, visit www.alpineimmunesciences.com. Follow @AlpineImmuneSci on X and LinkedIn.
Forward-Looking Statements
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. These forward-looking statements are not based on historical fact and include statements regarding our platform technology and potential therapies; the timing of and results from clinical trials and preclinical development activities; clinical and regulatory objectives and the timing thereof; expectations regarding the sufficiency of cash, including cash equivalents and restricted cash, and investments to fund our planned operations; our ability to achieve additional milestones in our collaborations and proprietary programs; the progress and potential of our development programs; future development plans and clinical and regulatory milestones and objectives, including the timing and achievement thereof; the efficacy of our clinical trial designs; anticipated enrollment in our clinical trials and the timing thereof; expectations regarding the anticipated reporting of data from our ongoing and planned clinical trials and potential publication of future clinical data; our ability to potentially advance povetacicept directly into a pivotal trial as well as a phase 2 study in systemic lupus erythematosus, and the potential efficacy, safety profile, addressable market, regulatory success and commercial or therapeutic potential of our product candidates. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions and include words such as “may,” “will,” “should,” “would,” “expect,” “plan,” “intend,” and other similar expressions, among others. These forward-looking statements are based on current assumptions that involve risks, uncertainties, and other factors that may cause actual results, events, or developments to be materially different from those expressed or implied by such forward-looking statements. These risks and uncertainties, many of which are beyond our control, include, but are not limited to: clinical trials may not demonstrate safety and efficacy of any of our product candidates; our ongoing discovery and preclinical efforts may not yield additional product candidates; our discovery stage and preclinical programs may not advance into the clinic or result in approved products; any of our product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; we may not achieve additional milestones in our proprietary or partnered programs; the impact of competition; adverse conditions in the general domestic and global economic markets; we may be unable to advance povetacicept directly into a pivotal trial or a phase 2 study in systemic lupus erythematosus; the impact of pandemics, or other related health crises on our business, research and clinical development plans and timelines and results of operations, including the impact on our clinical trial sites, collaborators, and contractors who act for or on our behalf; as well as the other risks identified in our filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof and we undertake no obligation to update forward-looking statements, and readers are cautioned not to place undue reliance on such forward-looking statements.
Additional Information about the Acquisition (the offer) of Alpine by Vertex Pharmaceuticals Incorporated (Vertex) and Where to Find It
The offer has not yet commenced. This press release is for informational purposes only, is not a recommendation and is neither an offer to purchase nor a solicitation of an offer to sell any securities, nor is it a substitute for the tender offer materials that Vertex and the Purchaser will file with the SEC upon commencement of the offer. A solicitation and offer to buy outstanding shares of Alpine will only be made pursuant to the tender offer materials that Vertex and the Purchaser intend to file with the SEC. At the time the offer is commenced, Vertex and the Purchaser will file with the SEC tender offer materials on Schedule TO, and Alpine will file with the SEC a Solicitation/Recommendation Statement on Schedule 14D-9 with respect to the offer.
THE TENDER OFFER MATERIALS (INCLUDING AN OFFER TO PURCHASE, A RELATED LETTER OF TRANSMITTAL AND CERTAIN OTHER TENDER OFFER DOCUMENTS) AND THE SOLICITATION/RECOMMENDATION STATEMENT WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION AND THE PARTIES THERETO. INVESTORS AND STOCKHOLDERS OF ALPINE ARE URGED TO READ THESE DOCUMENTS CAREFULLY WHEN THEY BECOME AVAILABLE (AND EACH AS IT MAY BE AMENDED OR SUPPLEMENTED FROM TIME TO TIME), BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION THAT INVESTORS AND STOCKHOLDERS OF ALPINE SHOULD CONSIDER BEFORE MAKING ANY DECISION REGARDING TENDERING THEIR SHARES OF COMMON STOCK IN THE OFFER. The tender offer materials (including the Offer to Purchase and the related Letter of Transmittal) will be made available to all stockholders of Alpine at no expense to them under the “Investors” section of Vertex’s website at https://investors.vrtx.com/financial-information/sec-filings or by email at Investorinfo@VRTX.com, or by directing requests for such materials to the information agent for the offer, which will be named in the tender offer materials, and (once they become available) the tender offer materials as well as the Solicitation/Recommendation Statement will be mailed to the stockholders of Alpine free of charge. Copies of the documents filed with the SEC by Alpine will be available free of charge on Alpine’s website, ir.alpineimmunesciences.com, or by contacting Alpine’s investor relations department at ir@alpineimmunesciences.com. The information contained in, or that can be accessed through, Vertex’s website and Alpine’s website is not a part of or incorporated by reference herein. The tender offer materials (including the Offer to Purchase and the related Letter of Transmittal), as well as the Solicitation/Recommendation Statement, will also be made available for free on the SEC’s website at www.sec.gov. In addition to the Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, Vertex and Alpine file annual, quarterly, and current reports, proxy statements, and other information with the SEC. You may read any reports, statements, or other information filed by Vertex and Alpine with the SEC for free on the SEC’s website at www.sec.gov.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240410724204/en/
Media and Investor Relations Contact:
Alpine Immune Sciences, Inc.
ir@alpineimmunesciences.com
media@alpineimmunesciences.com
Source: Alpine Immune Sciences, Inc.







