Aldeyra Therapeutics Achieves Primary Endpoint in Phase 3 Dry Eye Disease Clinical Trial of Reproxalap
Aldeyra Therapeutics (ALDX) has achieved the primary endpoint in a Phase 3 clinical trial of reproxalap for dry eye disease. The trial demonstrated statistically superior results for reproxalap over vehicle in reducing ocular discomfort, an FDA-accepted symptom of dry eye disease. This marks the first positive Phase 3 clinical trial in a dry eye chamber with a symptom as a primary endpoint.
The study involved 132 patients, with 66 receiving reproxalap and 66 receiving vehicle. Reproxalap was well-tolerated with no safety concerns. Aldeyra plans to resubmit its New Drug Application (NDA) in 2024, with an expected six-month FDA review period. The company believes these results support reproxalap's potential for rapid clinical effect in reducing ocular discomfort associated with dry eye disease.
Aldeyra Therapeutics (ALDX) ha raggiunto l'obiettivo primario in uno studio clinico di fase 3 su reproxalap per la malattia dell'occhio secco. Lo studio ha dimostrato risultati statisticamente superiori per reproxalap rispetto al placebo nella riduzione del disagio oculare, un sintomo accettato dalla FDA della malattia dell'occhio secco. Questo segna il primo studio clinico di fase 3 positivo in un contesto di occhio secco con un sintomo come obiettivo primario.
Lo studio ha coinvolto 132 pazienti, di cui 66 hanno ricevuto reproxalap e 66 hanno ricevuto il placebo. Reproxalap è stato ben tollerato e non ci sono state preoccupazioni per la sicurezza. Aldeyra prevede di ripresentare la propria domanda per l'Autorizzazione all'Immissione in Commercio (NDA) nel 2024, con un periodo di revisione previsto di sei mesi da parte della FDA. L'azienda ritiene che questi risultati supportino il potenziale di reproxalap per un effetto clinico rapido nella riduzione del disagio oculare associato alla malattia dell'occhio secco.
Aldeyra Therapeutics (ALDX) ha alcanzado el objetivo primario en un ensayo clínico de fase 3 de reproxalap para la enfermedad del ojo seco. El ensayo demostró resultados estadísticamente superiores para reproxalap en comparación con el placebo en la reducción del incomodidad ocular, un síntoma aceptado por la FDA de la enfermedad del ojo seco. Esto marca el primer ensayo clínico de fase 3 positivo en una cámara para el ojo seco con un síntoma como objetivo primario.
El estudio involucró a 132 pacientes, de los cuales 66 recibieron reproxalap y 66 recibieron placebo. Reproxalap se toleró bien sin preocupaciones de seguridad. Aldeyra planea volver a presentar su Solicitud de Nuevo Medicamento (NDA) en 2024, con un período de revisión esperado de seis meses por parte de la FDA. La empresa cree que estos resultados apoyan el potencial de reproxalap para un efecto clínico rápido en la reducción de la incomodidad ocular asociada con la enfermedad del ojo seco.
Aldeyra Therapeutics (ALDX)가 안구 건조증에 대한 reproxalap의 3상 임상 시험에서 주요 목표를 달성했습니다. 이 시험은 안구 건조증의 FDA 승인 증상인 안구 불편감을 줄이는 데 있어 reproxalap이 대조군보다 통계적으로 우수한 결과를 보였음을 입증했습니다. 이는 증상을 주요 종료목적으로 하는 첫 번째 긍정적인 3상 임상 시험입니다.
연구에는 132명의 환자가 참여했으며, 66명이 reproxalap를, 66명이 대조군을 받았습니다. Reproxalap는 잘 견뎌졌으며 안전성 우려가 없었습니다. Aldeyra는 2024년에 신약 승인 신청(NDA)을 재제출할 계획이며, FDA의 예상 리뷰 기간은 6개월입니다. 회사는 이러한 결과가 안구 건조증과 관련된 안구 불편감을 줄이는 데 있어 reproxalap의 빠른 임상적 효과를 지지한다고 믿습니다.
Aldeyra Therapeutics (ALDX) a atteint l'objectif principal d'un essai clinique de phase 3 portant sur reproxalap pour la maladie de l'œil sec. L'essai a montré des résultats statistiquement supérieurs pour reproxalap par rapport au placebo dans la réduction de l'inconfort oculaire, un symptôme accepté par la FDA de la maladie de l'œil sec. Cela marque le premier essai clinique de phase 3 positif dans une chambre de l'œil sec avec un symptôme en tant qu'objectif principal.
L'étude a impliqué 132 patients, dont 66 ont reçu reproxalap et 66 ont reçu le placebo. Reproxalap a été bien toléré sans préoccupations de sécurité. Aldeyra prévoit de soumettre à nouveau sa demande de nouveau médicament (NDA) en 2024, avec un délai de révision de six mois prévu par la FDA. L'entreprise croit que ces résultats soutiennent le potentiel de reproxalap pour un effet clinique rapide dans la réduction de l'inconfort oculaire associé à la maladie de l'œil sec.
Aldeyra Therapeutics (ALDX) hat das primäre Ziel in einer Phase-3-Studie zu reproxalap bei Trockenheitskrankheiten des Auges erreicht. Die Studie zeigte statistisch überlegene Ergebnisse für reproxalap im Vergleich zu einem Placebo bei der Reduzierung von ocular discomfort, einem von der FDA akzeptierten Symptom der Trockenheitskrankheit des Auges. Dies markiert die erste positive Phase-3-Studie in einer Trockenaugensituation mit einem Symptom als primäres Ziel.
In der Studie waren 132 Patienten beteiligt, von denen 66 reproxalap und 66 ein Placebo erhielten. Reproxalap wurde gut vertragen, ohne Sicherheitsbedenken. Aldeyra plant, 2024 seinen Antrag auf Zulassung eines neuen Arzneimittels (NDA) erneut einzureichen, wobei mit einer sechsmonatigen Überprüfungszeit durch die FDA zu rechnen ist. Das Unternehmen glaubt, dass diese Ergebnisse das Potenzial von reproxalap für eine schnelle klinische Wirkung bei der Reduzierung von ocular discomfort im Zusammenhang mit Trockenheit des Auges unterstützen.
- Achieved primary endpoint in Phase 3 clinical trial for dry eye disease
- Statistically superior results in reducing ocular discomfort compared to vehicle (P=0.004)
- First positive Phase 3 clinical trial in a dry eye chamber with a symptom as primary endpoint
- Well-tolerated with no safety concerns in over 2,500 patients studied
- Potential for rapid clinical effect in reducing ocular discomfort
- NDA resubmission not expected until 2024
- Previously received complete response letter from FDA requiring additional study
Insights
Aldeyra's announcement of achieving the primary endpoint in their Phase 3 dry eye disease trial for reproxalap is a significant milestone. The study's success in demonstrating statistical superiority over vehicle for ocular discomfort (P=
This result potentially positions reproxalap as a first-in-class treatment with both acute and chronic efficacy in symptom reduction and ocular redness improvement. The planned NDA resubmission in 2024 could lead to a 6-month review period, potentially bringing this novel treatment to market relatively soon.
However, investors should note that while promising, FDA approval is not guaranteed. The company's claim of being the first to achieve positive Phase 3 results in a dry eye chamber with a symptom as the primary endpoint needs verification. If confirmed, it could significantly differentiate reproxalap in the competitive dry eye disease market.
The Phase 3 trial design for reproxalap demonstrates robust methodology, aligning with FDA guidance. The use of a vehicle-controlled, randomized, double-masked study with 132 patients (66 per arm) provides a solid foundation for data interpretation. The primary endpoint of ocular discomfort from 80 to 100 minutes in a dry eye chamber is a clinically relevant measure.
Importantly, the trial's design to satisfy FDA's requirement for an "additional adequate and well-controlled study" through the Special Protocol Assessment process enhances the likelihood of regulatory acceptance. The absence of safety signals and good tolerability profile, consistent with previous studies involving over 2,500 patients, further strengthens reproxalap's potential.
However, it's important to await peer-reviewed publication of full trial results to assess the magnitude of effect and durability of response, which will be key factors in determining reproxalap's clinical significance and market potential.
Aldeyra's positive Phase 3 results for reproxalap could significantly impact the dry eye disease market, currently valued at over
The company's stock (NASDAQ: ALDX) is likely to see increased investor interest following this news. However, it's important to consider that Aldeyra is a small-cap biotech with a narrow pipeline, making it susceptible to high volatility based on clinical trial outcomes and regulatory decisions.
While the potential 2024 NDA resubmission and 6-month review period suggest a relatively near-term catalyst, investors should be aware of the risks associated with the FDA approval process. The dry eye disease market is competitive and successful commercialization will require significant resources. Aldeyra's ability to effectively launch and market reproxalap, if approved, will be important for long-term value creation.
New Drug Application Resubmission Anticipated in 2024
(Graphic: Aldeyra Therapeutics)
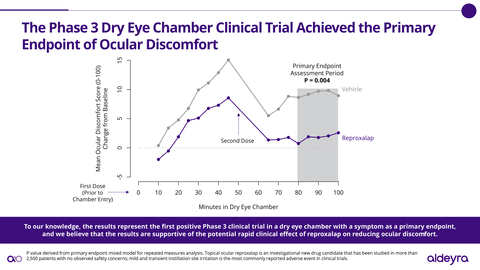
“To our knowledge, the results announced today represent the first positive Phase 3 clinical trial in a dry eye chamber with a symptom as a primary endpoint, and we believe that the results are supportive of the potential rapid clinical effect of reproxalap on reducing the ocular discomfort associated with dry eye disease,” stated Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer of Aldeyra.
In the Phase 3 clinical trial, patients were administered vehicle (the drug product without the active ingredient) before and during exposure to a dry eye chamber in a manner that Aldeyra believes is consistent with the FDA’s dry eye disease draft guidance1. Qualifying patients were subsequently randomized to receive either reproxalap or vehicle before and during exposure to an additional dry eye chamber. Of the 132 patients randomized, 66 patients received reproxalap and 66 patients received vehicle. The primary endpoint was ocular discomfort, an FDA-accepted symptom of dry eye disease, from 80 to 100 minutes in the chamber. The dry eye chamber clinical trial was designed to satisfy the FDA’s New Drug Application (NDA) resubmission requirement, identified in the previously received complete response letter, of “at least one additional adequate and well-controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye.” Through the FDA Special Protocol Assessment process and additional comments, the FDA provided feedback on the clinical trial protocol and statistical plan.
To Aldeyra’s knowledge, in patients with dry eye disease, reproxalap is the first investigational drug with pivotal data supportive of acute and chronic activity in reducing symptoms, and the first investigational drug for chronic administration with pivotal data supportive of acute activity in reducing ocular redness. The potential NDA resubmission is anticipated in 2024. Based on FDA guidance, the resubmission NDA review period is expected to be six months.
There were no safety signals observed in the clinical trial, and reproxalap was observed to be well tolerated. Consistent with prior clinical trials, the most commonly reported adverse event was mild and transient instillation site discomfort. No treatment-related discontinuations were reported. Reproxalap has now been studied in over 2,500 patients.
Conference Call & Webcast Information
Aldeyra will host a conference call at 9:00 a.m. ET today, August 8, 2024, to discuss the Phase 3 dry eye chamber trial results and the plan for resubmission of the NDA for reproxalap in dry eye disease. The dial-in numbers are (888) 596-4144 for domestic callers and (646) 968-2525 for international callers. The access code is 7321123. A live webcast of the conference call will be available on the Investor Relations page of the company’s website at https://ir.aldeyra.com. After the live webcast, the event will remain archived on the Aldeyra Therapeutics website for 90 days.
About Aldeyra
Aldeyra Therapeutics is a biotechnology company devoted to discovering innovative therapies designed to treat immune-mediated and metabolic diseases. Our approach is to develop pharmaceuticals that modulate protein systems, instead of directly inhibiting or activating single protein targets, with the goal of optimizing multiple pathways at once while minimizing toxicity. Our product candidates include RASP (reactive aldehyde species) modulators ADX-629, ADX-248, ADX-743, ADX-631, and chemically related molecules for the potential treatment of systemic and retinal immune-mediated and metabolic diseases. Our late-stage product candidates are reproxalap, a RASP modulator for the potential treatment of dry eye disease and allergic conjunctivitis, and ADX-2191, a novel formulation of intravitreal methotrexate for the potential treatment of retinitis pigmentosa.
About Reproxalap
Reproxalap is an investigational new drug candidate in development for the treatment of dry eye disease and allergic conjunctivitis, two of the largest markets in ophthalmology. Reproxalap is a first-in-class small-molecule modulator of RASP, which are elevated in ocular and systemic inflammatory diseases. The mechanism of action of reproxalap has been supported by the demonstration of statistically significant and clinically relevant activity in multiple physiologically distinct late-phase clinical indications. Reproxalap has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials.
Safe Harbor Statement
This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Aldeyra’s future expectations, plans, and prospects, including without limitation statements regarding: the goals, opportunity, and potential for reproxalap; the outcome and timing of the FDA’s review, acceptance and/or approval of a potential NDA resubmission for reproxalap and the adequacy of the data included in the original NDA and such NDA resubmission; and Aldeyra’s expectations regarding the labeling for reproxalap, if approved. Aldeyra intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” "could," “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “on track,” “scheduled,” “target,” “design,” “estimate,” “predict,” “contemplates,” “likely,” “potential,” “continue,” “ongoing,” “aim,” “plan,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. Aldeyra is at an early stage of development and may not ever have any products that generate significant revenue. All of Aldeyra's development timelines may be subject to adjustment depending on recruitment rate, regulatory review, preclinical and clinical results, funding, and other factors that could delay the initiation, enrollment, or completion of clinical trials. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements include, among others, the timing of enrollment, commencement and completion of Aldeyra's clinical trials, the timing and success of preclinical studies and clinical trials conducted by Aldeyra and its development partners; delay in or failure to obtain regulatory approval of Aldeyra's product candidates, including as a result of the FDA not accepting Aldeyra’s regulatory filings, issuing a complete response letter, or requiring additional clinical trials or data prior to review or approval of such filings or in connection with resubmissions of such filings; the ability to maintain regulatory approval of Aldeyra's product candidates, and the labeling for any approved products; the risk that prior results, such as signals of safety, activity, or durability of effect, observed from preclinical or clinical trials, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Aldeyra's product candidates in clinical trials focused on the same or different indications; the scope, progress, expansion, and costs of developing and commercializing Aldeyra's product candidates; uncertainty as to Aldeyra’s ability to commercialize (alone or with others) and obtain reimbursement for Aldeyra's product candidates following regulatory approval, if any; the size and growth of the potential markets and pricing for Aldeyra's product candidates and the ability to serve those markets; Aldeyra's expectations regarding Aldeyra's expenses and future revenue, the timing of future revenue, the sufficiency or use of Aldeyra's cash resources and needs for additional financing; the rate and degree of market acceptance of any of Aldeyra's product candidates; Aldeyra's expectations regarding competition; Aldeyra's anticipated growth strategies; Aldeyra's ability to attract or retain key personnel; Aldeyra’s commercialization, marketing and manufacturing capabilities and strategy; Aldeyra's ability to establish and maintain development partnerships; Aldeyra’s ability to successfully integrate acquisitions into its business; Aldeyra's expectations regarding federal, state, and foreign regulatory requirements; political, economic, legal, social, and health risks, public health measures, and war or other military actions, that may affect Aldeyra’s business or the global economy; regulatory developments in
In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this release is provided only as of the date of this release, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
1 www.fda.gov/media/144594/download
View source version on businesswire.com: https://www.businesswire.com/news/home/20240808408806/en/
Investor & Media:
David Burke
Tel: (917) 618-2651
investorrelations@aldeyra.com
Source: Aldeyra Therapeutics, Inc.
FAQ
What was the primary endpoint of Aldeyra's Phase 3 trial for reproxalap (ALDX)?
How many patients were involved in Aldeyra's Phase 3 trial for reproxalap (ALDX)?
When does Aldeyra Therapeutics (ALDX) plan to resubmit its New Drug Application for reproxalap?







