Akebia Presents Results from its INNO2VATE Global Phase 3 Program; Demonstrated Efficacy and Cardiovascular Safety of Vadadustat for the Treatment of Anemia due to Chronic Kidney Disease in Adult Patients on Dialysis
Akebia Therapeutics (Nasdaq: AKBA) presented data from its INNO2VATE Phase 3 program at ASN Kidney Week 2020, highlighting the efficacy and cardiovascular safety of vadadustat for treating anemia in adult dialysis patients. The results showed vadadustat's non-inferiority to darbepoetin alfa across key metrics, including major adverse cardiovascular events (MACE). Akebia aims to submit a New Drug Application (NDA) to the FDA in 2021 and plans to present additional trial results at ASN Kidney Week.
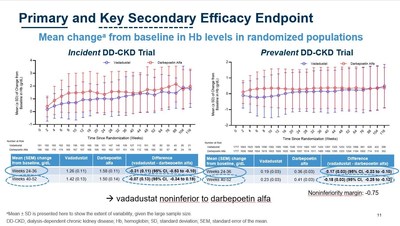
- Vadadustat demonstrated non-inferiority to darbepoetin alfa in mean hemoglobin change, achieving predefined efficacy endpoints.
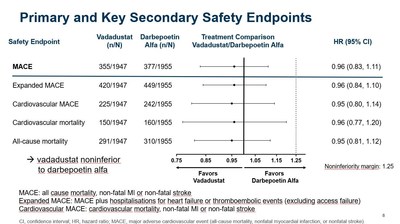
- Presented data reinforced vadadustat's cardiovascular safety profile, showing non-inferiority in MACE and all-cause mortality.
- Successful outcomes across diverse patient populations enhance the potential for vadadustat's approval as a new oral treatment option.
- None.
Insights
Analyzing...
CAMBRIDGE, Mass., Oct. 22, 2020 /PRNewswire/ -- Akebia Therapeutics, Inc. (Nasdaq: AKBA) today announced the presentation of clinical data from its global INNO2VATE Phase 3 program, which demonstrated the efficacy and cardiovascular safety of vadadustat for the treatment of anemia due to chronic kidney disease (CKD) in adult patients on dialysis, at American Society of Nephrology Kidney Week 2020 Reimagined (ASN Kidney Week). Vadadustat is Akebia's investigational oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) in development for the treatment of anemia due to CKD.
"The data presented today build on the positive top-line efficacy and safety results from INNO2VATE that were previously reported in May. More specifically, vadadustat's cardiovascular safety profile in dialysis patients is further reinforced by newly presented data clearly showing vadadustat achieved non-inferiority to darbepoetin alfa on MACE, expanded MACE, cardiovascular MACE, cardiovascular mortality, and all-cause mortality. These results were also consistent across multiple pre-specified populations," said Kai-Uwe Eckardt, M.D., Professor of Medicine and Head of the Department of Nephrology and Medical Intensive Care Medicine at the Charité in Berlin and Co-Chair of the independent Executive Steering Committee for INNO2VATE. "Left untreated, anemia in dialysis patients results in high transfusion requirements and severely reduces a patient's quality of life. The INNO2VATE results demonstrate that vadadustat could represent an attractive new oral treatment option for patients new to and already established on dialysis, upon approval."
Results from INNO2VATE are being presented today at ASN Kidney Week during a presentation titled, "Global Phase 3 Clinical Trials of Vadadustat vs Darbepoetin Alfa for Treatment of Anemia in Patients with Dialysis-Dependent Chronic Kidney Disease" (Abstract TH-OR01).
Highlights of the INNO2VATE ASN Kidney Week Presentation:
Efficacy:
- As previously reported, vadadustat achieved the primary and key secondary efficacy endpoints in each of the two INNO2VATE studies, which demonstrated non-inferiority to darbepoetin alfa as measured by a mean change in hemoglobin (Hb) between baseline and the primary evaluation period (weeks 24 to 36) and secondary evaluation period (weeks 40 to 52). Non-inferiority was achieved as the lower bound of the
Safety:
- As previously reported, vadadustat achieved the primary safety endpoint of the INNO2VATE program, defined as non-inferiority of vadadustat versus darbepoetin alfa in time to first occurrence of a major adverse cardiovascular event (MACE), which is the composite of all-cause mortality, non-fatal myocardial infarction (MI), or non-fatal stroke across both INNO2VATE studies.
- INNO2VATE results on key secondary safety endpoints were clear and consistent. Vadadustat demonstrated non-inferiority to darbepoetin alfa in analyses of expanded MACE, cardiovascular MACE, cardiovascular mortality, and all-cause mortality.
- The incidence of treatment emergent adverse events during the incident dialysis patient (Correction/Conversion) study in vadadustat treated patients was
"We could not be more excited and pleased with such compelling results across our clinical development program in patients on dialysis for vadadustat," said John P. Butler, President and CEO of Akebia Therapeutics. "The clear and consistent data set the stage for the potential approval of vadadustat and underscore its potential as a new oral standard of care for treating patients on dialysis with anemia due to CKD, including both incident and prevalent dialysis patients, upon approval. We look forward to bringing this innovative therapy to patients on dialysis globally, upon approval, and remain on track to submit an NDA as early as possible next year."
Akebia's vadadustat development program also includes PRO2TECT, the global Phase 3 program for the treatment of anemia due to CKD in adult patients not on dialysis. Results from this program will be presented at ASN Kidney Week in a late-breaking presentation on October 23, 2020. Akebia plans to submit to the U.S. Food and Drug Administration (FDA) a New Drug Application (NDA) for vadadustat for the treatment of anemia due to CKD in adult dialysis-dependent and non-dialysis dependent patients as early as possible in 2021. In addition, Akebia and its collaborator, Otsuka Pharmaceutical Co. Ltd., are working in close collaboration to prepare a Marketing Authorization Application (MAA) for submission to the European Medicines Agency (EMA) next year.
Investor Briefing Webcast
Akebia management will host an investor briefing webcast with Dr. Glenn Chertow, M.D., M.P.H., Co-Chair of the independent Executive Steering Committee for PRO2TECT and INNO2VATE, on October 23, 2020 at 4:10 p.m. ET to review highlights of the Phase 3 vadadustat data presentations from ASN Kidney Week. To access Akebia's investor briefing webcast and the accompanying slides please log into the Investors section of the Company's website at https://ir.akebia.com and proceed to the events and presentations page at https://ir.akebia.com/events-and-presentations. Please connect to the Company's website at least 10 minutes prior to the online event to ensure adequate time for any software download that may be required to view the webcast. After the webcast concludes, a replay of the event will be available at that same location until October 29, 2020.
About the INNO2VATE Global Phase 3 Program of Vadadustat
Akebia's global INNO2VATE program included two separate Phase 3 studies for incident dialysis patients (Correction/Conversion) and prevalent dialysis patients (Conversion), which collectively enrolled 3,923 adult patients on dialysis with anemia due to CKD. The INNO2VATE incident dialysis patient study evaluated 369 incident dialysis patients and the prevalent dialysis study evaluated 3,554 prevalent dialysis patients. Both INNO2VATE studies were global, multicenter, open-label, sponsor-blind, active-controlled (darbepoetin alfa - an injectable erythropoiesis stimulating agent), non-inferiority studies. In both studies, patients were randomized 1:1 to receive either oral vadadustat or injectable darbepoetin alfa. Efficacy and safety results were measured against non-inferiority margins agreed upon with the FDA and the EMA.
About Anemia due to Chronic Kidney Disease (CKD)
Anemia is a condition in which a person lacks enough healthy red blood cells to carry adequate oxygen to the body's tissues. It commonly occurs in people with CKD because their kidneys do not produce enough erythropoietin (EPO), a hormone that helps regulate production of red blood cells. Anemia due to CKD can have a profound impact on a person's quality of life as it can cause fatigue, dizziness, shortness of breath and cognitive dysfunction. Left untreated, anemia leads to deterioration in health and is associated with increased morbidity and mortality in people with CKD.
About Vadadustat
Vadadustat is an oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor designed to mimic the physiologic effect of altitude on oxygen availability. At higher altitudes, the body responds to lower oxygen availability with stabilization of hypoxia-inducible factor, which can lead to increased red blood cell production and improved oxygen delivery to tissues. Vadadustat is in global Phase 3 development for the treatment of anemia due to CKD and is not approved by the U.S. Food and Drug Administration (FDA) or any regulatory authority with the exception of Japan's Ministry of Health, Labour and Welfare (MHLW). In Japan, vadadustat is approved as a treatment for anemia due to CKD in both dialysis-dependent and non-dialysis dependent adult patients.
About Akebia Therapeutics
Akebia Therapeutics, Inc. is a fully integrated biopharmaceutical company with the purpose to better the lives of people impacted by kidney disease. The Company was founded in 2007 and is headquartered in Cambridge, Massachusetts. For more information, please visit our website at www.akebia.com, which does not form a part of this release.
Forward Looking Statements
Statements in this press release regarding Akebia's strategy, plans, prospects, expectations, beliefs, intentions and goals are forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, including but not limited to statements regarding establishing vadadustat as a treatment option and a new oral standard of care for treating patients on dialysis with anemia due to CKD; the potential for obtaining approval of vadadustat in dialysis; safety and efficacy of vadadustat in dialysis; the potential indications for and benefits of vadadustat; submitting filings for marketing approval of vadadustat, and the timing thereof; and the clinical opportunity for vadadustat. The terms "could," "look forward," "on track," "plan," "potential," "will," "working" and similar references are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement, including the timing and content of advice given and decisions made by health authorities, including approval and labeling decisions; the actual time it takes to make regulatory submissions for vadadustat to health authorities, including the submission of the NDA to the FDA and the submission of the MAA to the EMA; risks associated with the Priority Review Voucher for vadadustat; the potential direct or indirect impact of the COVID-19 pandemic on our business, operations, and the markets and communities in which we and our partners, collaborators, vendors and customers operate; manufacturing and quality risks; risks associated with management and key personnel changes and transitional periods; the actual funding required to continue to commercialize our commercial product, to develop and commercialize vadadustat, and to operate the Company; market acceptance and coverage and reimbursement of our commercial product and vadadustat, if approved; the risks associated with potential generic entrants for our commercial product and vadadustat, if approved; early termination of any of Akebia's collaborations; Akebia's and its collaborators' ability to satisfy their obligations under Akebia's collaboration agreements; the competitive landscape for our commercial product and vadadustat; the scope, timing, and outcome of any legal, regulatory and administrative proceedings; changes in the economic and financial conditions of the businesses of Akebia and its collaborations partners and vendors; and Akebia's ability to obtain, maintain and enforce patent and other intellectual property protection for our commercial product, vadadustat and any other product candidates. Other risks and uncertainties include those identified under the heading "Risk Factors" in Akebia's Quarterly Report on Form 10-Q for the quarter ended June 30, 2020 and other filings that Akebia may make with the U.S. Securities and Exchange Commission in the future. These forward-looking statements (except as otherwise noted) speak only as of the date of this press release, and Akebia does not undertake, and specifically disclaims, any obligation to update any forward-looking statements contained in this press release.
Investor Contact
Kristen K. Sheppard, Esq.
Ir@akebia.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-inno2vate-global-phase-3-program-demonstrated-efficacy-and-cardiovascular-safety-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-on-dialysis-301158444.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-inno2vate-global-phase-3-program-demonstrated-efficacy-and-cardiovascular-safety-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-on-dialysis-301158444.html
SOURCE Akebia Therapeutics