Ainos, Nisshinbo Micro Devices and Inabata Initiate Phase 2 of VOC Co-development Powered by AI Nose, Accelerating the Digitalization of Smell
- None.
- None.
Insights
The announcement by Ainos, Inc. regarding the advancement to the second phase of co-development for their AI Nose technology indicates a strategic move towards diversifying applications and expanding market reach. This technological innovation, which involves the detection and analysis of volatile organic compounds (VOCs), has significant implications for multiple sectors, including healthcare, automotive, industrial and environmental safety.
From an industry perspective, the collaboration with Nisshinbo Micro Devices Inc. and Taiwan Inabata Sangyo Co. is pivotal in refining the technology's functionality and ensuring its compatibility with various market needs. The focus on key hardware and software aspects, such as manufacturing processes, user interface and safety requirements, is crucial for the successful adoption of the AI Nose technology.
The potential benefits of this technology are substantial, as it could revolutionize point-of-care testing and preventative medicine by providing real-time, non-invasive diagnostic capabilities. Additionally, in the automotive and industrial sectors, the ability to detect harmful VOCs could enhance safety and environmental monitoring. The long-term impact on stakeholders could include opening new revenue streams, improving product safety and contributing to the overall growth of the VOC sensing market.
However, challenges such as regulatory approval, market acceptance and the scalability of mass production remain. It is also important to consider the competitive landscape, as other companies may develop similar technologies. The successful commercialization will depend on the platform's accuracy, reliability and cost-effectiveness compared to existing solutions.
The market's response to Ainos' announcement is likely to hinge on the company's ability to meet its targeted mass production readiness date in the second half of 2024. Investors will be monitoring the progress closely, as delays or advancements could influence the company's stock performance. The co-development also highlights the strategic use of Ainos' intellectual property portfolio, which includes over 50 active and pending patents, potentially strengthening its competitive position and future revenue potential.
Financially, the investment in R&D and the subsequent mass production phase could impact Ainos' short-term cash flow. However, if successful, the long-term returns could be significant, given the broad application potential of the technology. Investors should also consider the company's ability to secure additional partnerships or funding to support the development and commercialization of the AI Nose platform.
It is essential for investors to evaluate the company's track record in innovation and its capability to navigate the complex regulatory environment that accompanies healthcare-related technologies. The announcement does not provide specific financial projections, so stakeholders should seek further information regarding the expected impact on the company's financials once the prototype is finalized and production scales up.
The integration of AI Nose technology within the telehealth sector could signify a major advancement in remote patient monitoring and diagnostics. The ability to accurately detect and analyze VOCs through non-invasive means could lead to earlier detection of diseases and better patient outcomes. This aligns with the ongoing trend towards personalized and precision medicine.
Medically, the success of Ainos' AI Nose technology depends on its clinical validation and the establishment of a robust, evidence-based framework for its use in diagnostics. The medical community will scrutinize the platform's sensitivity, specificity and overall diagnostic accuracy. Additionally, the ability to integrate with existing healthcare systems and protocols will be critical for adoption.
For stakeholders in the healthcare industry, including hospitals, clinics and insurance companies, the implications of a successful AI Nose technology could be transformative. It could lead to cost savings through reduced need for more invasive diagnostic procedures and potential for home-based monitoring solutions. However, the adoption curve may be gradual, as medical professionals will require training and time to adapt to the new technology.
Mass Production Readiness Targeted for 2H 2024
Propelling AI Nose Technology Towards Broader Industry Adoption and Advancing the Mission of Digitalizing Smell
SAN DIEGO, CA / ACCESSWIRE / December 26, 2023 / Ainos, Inc. (NASDAQ: AIMD, AIMDW) ("Ainos", or the "Company"), a diversified healthcare company focused on the development of novel point-of-care testing, low-dose interferon therapeutics, and synthetic RNA-driven preventative medicine, today announced that it is initiating the second phase of co-development of a volatile organic compound (VOC) sensing platform, powered by AI Nose technology, in collaboration with Nisshinbo Micro Devices Inc. (NISD) and Taiwan Inabata Sangyo Co. (Inabata). The parties aim to finalize the prototype in the third quarter of 2024 and subsequently prepare for mass production.
"I'm thrilled to say that Ainos, NISD and Inabata are initiating the second phase just four months after our initial co-development announcement in August 2023. This rapid progress underscores our unwavering commitment to digitizing smell by pioneering VOC sensing's potential across diverse industries. The collaboration signals an era of innovation for our AI Nose technology, expanding the potential application of our AI-powered VOC sensing technology to telehealth, automotive, industrial, and environmental safety, thereby broadening our addressable market. We look forward to successfully launching the product in the second half of 2024," said Chun-Hsien (Eddy) Tsai, Chairman, President and CEO of Ainos.
In the second phase, the parties plan to finalize the platform's functionality, specifications, and market positioning for the platform. In addition, the parties will also focus on developing the solution's key hardware and software aspects, including manufacturing, form factor, user interface, and safety requirements.
Under the co-development, Ainos and NISD will co-develop a VOC sensing platform that leverages Ainos' digital nose intellectual properties, the AI Nose platform. The co-developed platform is intended to be used in a variety of applications including telehealth, automotive, industrial, and environmental safety. Inabata will provide business logistics for the program and liaise between Ainos and NISD.
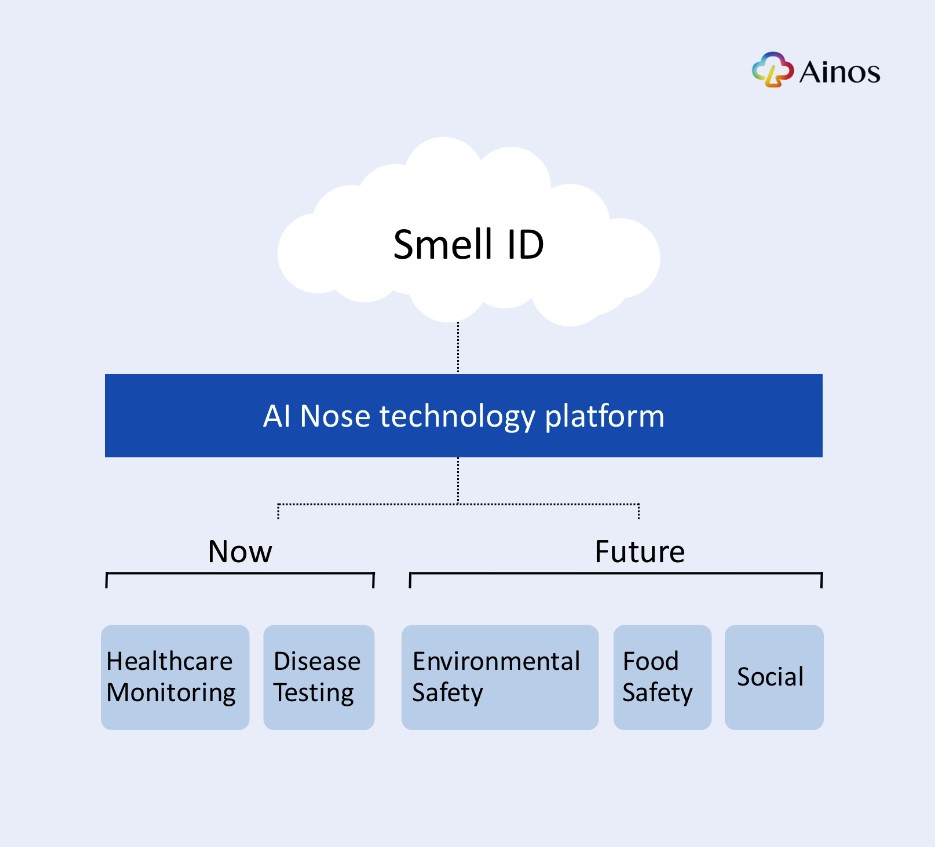
The Parties have a shared vision for the future of VOC sensing technology across a variety of applications, including telehealth, automotive, industrial, and environmental safety. To achieve this vision, the Parties have committed to collaborating on the development of innovative technologies by leveraging Ainos' cutting-edge artificial intelligence (AI) algorithm and digital nose technologies. These technologies have been developed over the past decade, and are protected by over 50 active and pending patents. By combining their expertise and resources, the Parties aim to create a new standard in VOC sensing and analysis, utilizing Ainos' deep-learning algorithm and cloud-based big data analysis to provide unparalleled accuracy and insight.
About Ainos, Inc.
Headquartered in San Diego, California, Ainos, Inc. is a diversified healthcare company engaged in developing innovative medical technologies for point-of-care testing and safe and novel medical treatment for a broad range of disease indications. In addition to its proprietary therapeutics using low-dose non-injectable interferon, Ainos has also expanded its product portfolio to include Volatile Organic Compounds (VOC) and COVID-19 POCTs. Powered by its AI Nose platform, the lead POCT candidate, Ainos Flora, is a telehealth-friendly POCT for women's health and certain common STIs. To learn more, visit https://www.ainos.com.
Follow Ainos on X, formerly known as Twitter, (@AinosInc) and LinkedIn to stay up-to-date.
About Nisshinbo Micro Devices Inc.
Nisshinbo Micro Devices Inc. provides analog solutions through electronic devices and microwave products. The company's comprehensive analog solutions serve a broad range of industries including automotives, industrials and consumers. It is the result of an integration of former companies New Japan Radio Co., Ltd. and RICOH Electronic Devices Co., Ltd. The company employs approximately 1,920 people. Nisshinbo Micro Devices Inc. is headquartered in Tokyo, Japan. For more information, visit: https://www.nisshinbo-microdevices.co.jp/en/.
About Taiwan Inabata Sangyo Co., Ltd.
Founded in 1989, Taiwan Inabata Sangyo Co., Ltd is a fully owned Taiwanese subsidiary of Inabata & Co. Ltd. The parent company Inabata & Co. Ltd. is a multifaceted trading company operating in four business segments: Information & Electronics, Plastics, Chemicals and Life Industry. Inabata & Co. Ltd. operates in about 60 locations in 18 countries. Inabata & Co. Ltd.is headquartered in Osaka Japan and is listed in Tokyo Stock Exchange under stock symbol 8098. Inabata is founded in 1890. Taiwan Inabata Sangyo Co., Ltd has devoted to grow with the innovators by helping them to connect business within the Inabata group. It traditionally focused on the trading of display related materials and equipment, semiconductor materials, engineering plastics, industrial chemicals but it has expanded coverage to the gas sensor industry, and healthcare industry in lien with parent company's strategy. For more information, visit: https://www.inabata.co.jp/english/.
Forward-Looking Statements
This press release contains "forward-looking statements" about Ainos within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by the use of words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict," "project," "target," "future," "likely," "strategy," "foresee," "may," "guidance," "potential," "outlook," "forecast," "should," "will" or other similar words or phrases. Similarly, statements that describe the Company's objectives, plans or goals are, or may be, forward-looking statements. Forward-looking statements are based only on the Company's current beliefs, expectations, and assumptions. Forward-looking statements are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of the Company's control. The Company's actual results may differ materially from those indicated in the forward-looking statements.
Important factors that could cause the Company's actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release include, among others, the cost of production and sales potential of the planned drug treatments announced in this press release; the Company's dependence on revenues from the sale of COVID-19 test kits and VELDONA Pet supplements; the Company's limited cash and history of losses; the Company's ability to achieve profitability; the Company's ability to raise additional capital to continue the Company's product development; the ability to accurately predict the future operating results of the Company; the ability to advance Ainos' current or future product candidates through clinical trials, obtain marketing approval and ultimately commercialize any product candidates the Company develops; the ability to obtain and maintain regulatory approval of Ainos product candidates; delays in completing the development and commercialization of the Company's current and future product candidates, which could result in increased costs to the Company, delay or limit the ability to generate revenue and adversely affect the business, financial condition, results of operations and prospects of the Company; intense competition and rapidly advancing technology in the Company's industry that may outpace its technology; customer demand for the products and services the Company develops; the impact of competitive or alternative products, technologies and pricing; disruption in research and development facilities; lawsuits and other claims by third parties or investigations by various regulatory agencies governing the Company's operations; potential cybersecurity attacks; increased requirements and costs related to cybersecurity; the Company's ability to realize the benefits of third party licensing agreements; the Company's ability to obtain and maintain intellectual property protection for Ainos product candidates; compliance with applicable laws, regulations and tariffs; and the Company's success in managing the growth. A more complete description of these risk factors and others is included in the "Risk Factors" section of Ainos' most recent Annual Report on Form 10-K/A and other reports filed with the U.S. Securities and Exchange Commission, many of which risks are beyond the Company's control. In addition to the risks described above, and in the Company's Annual Report on Form 10-K/A, other unknown or unpredictable factors also could cause actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release.
The forward-looking statements made in this press release are expressly qualified in their entirety by the foregoing cautionary statements. Ainos undertakes no obligation to, and expressly disclaims any such obligation to, publicly update or revise any forward-looking statement to reflect changed assumptions, the occurrence of anticipated or unanticipated events or changes to the future results over time or otherwise, except as required by law.
Investor Relations Contact
ICR, LLC
Robin Yang
Tel: +1 646-224-6971
Email: Ainos.IR@icrinc.com
SOURCE: Ainos, Inc.
View the original press release on accesswire.com
FAQ
What is the latest announcement from Ainos, Inc. (AIMD, AIMDW)?
What is the goal of the collaboration between Ainos, Nisshinbo Micro Devices Inc., and Taiwan Inabata Sangyo Co.?
What industries will benefit from the AI Nose technology developed by Ainos, Nisshinbo Micro Devices Inc., and Taiwan Inabata Sangyo Co.?
What aspects will be focused on in the second phase of co-development?







