Two Large Studies Demonstrate Complete Revascularization with Impella Heart Pumps Improves Ejection Fraction and Long-Term Patient Outcomes
“The contemporary data from these two prospective studies provide evidence that the adoption of Impella best practices is improving safety and reducing MACCE in the high-risk PCI patient population,” said
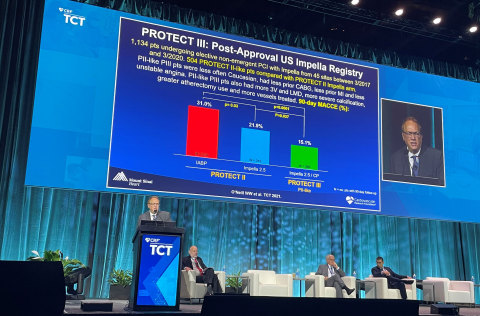
PROTECT III Final Results
The PROTECT III prospective study demonstrates improvement in 90-day clinical outcomes, completeness of revascularization, and safety, when compared to the PROTECT II Randomized Controlled Trial (RCT). The PROTECT II RCT found, when compared to intra-aortic balloon pump (IABP), Impella use led to a
Study authors analyzed patients in PROTECT III who would have qualified for PROTECT II, known as “PII-like” patients, and compared them to PROTECT II patients. PROTECT III patients had improved 90-day MACCE rates, compared to PROTECT II patients (
The study authors also note that PROTECT III patients, when compared to patients in PROTECT II:
- Were more complex, with more severe calcification, more rotational atherectomy and more vessels treated.
-
Had more complete revascularization, with
78% less hypotension during support (2.2% vs.10.2% , p=0.0004). -
Had improved in-hospital safety, with significantly fewer bleeding complications requiring transfusion (
1.2% vs.9.4% , p<0.001).
Restore EF Final Results
The Restore EF prospective study demonstrates the use of contemporary best practices with Impella in high-risk PCI significantly improves left ventricular ejection fraction (LVEF), heart failure symptoms, and anginal symptoms at 90-day follow-up in a wide variety of hospital settings including rural, urban, community and academic centers.
The study of 251 patients at 26 hospitals showed:
-
Significant improvement in LVEF from baseline to 90-day follow-up (
35% to45% p<0.0001). LVEF improvement at 90 days is the study’s primary endpoint. Restore EF is the latest study in a growing body of evidence demonstrating LVEF improvement with Impella-supported high-risk PCI. (see figure 2) -
Significant reduction of heart failure symptoms with
76% reduction inNew York Heart Association (NYHA) classification III/IV at follow-up (62% to15% p<0.001). (see figure 3) -
Significant reduction of anginal symptoms with
97% reduction inCanadian Cardiovascular Society (CCS) classification III/IV at follow-up (72% to2% p<0.0001). (see figure 3)
Advancement in Technology and Best Practices
Since PROTECT II, Impella-supported high-risk PCI has evolved to include the more powerful Impella CP with SmartAssist heart pump, which was used in about two-thirds of the patients in the Restore EF and PROTECT III studies.
“Advancement in technology, along with best practice learnings and operator experience has led to improvements in patient outcomes in contemporary practice,” said
The PROTECT series of studies and the Restore EF study are sponsored by
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are
The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5® with SmartAssist® are
ABOUT
Based in
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in
View source version on businesswire.com: https://www.businesswire.com/news/home/20211104005652/en/
For further information:
Media Contact:
Director of Communications
+1 (978) 882-8408
tlangford@abiomed.com
Investor:
Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com
Source:







