Multi-Center, Multi-Society Study of Impella-supported Patients with Cardiogenic Shock due to Myocarditis in Japan Achieves 30-day Survival of 77%
Abiomed (ABMD) announced a 30-day survival rate of 77% for patients with cardiogenic shock due to myocarditis, based on a three-year study at 109 hospitals in Japan. This represents a significant improvement over the 48% survival rate for those receiving only VA ECMO support. The findings were presented at the 2022 Transcatheter Cardiovascular Therapeutics conference. The study involved 143 patients and was conducted by the J-PVAD registry, highlighting the potential of Impella support in enhancing heart recovery amidst rising myocarditis cases linked to COVID-19.
- 77% 30-day survival rate for myocarditis patients using Impella support.
- Improvement from 48% survival rate with VA ECMO only.
- Study conducted at 109 hospitals under the J-PVAD registry.
- None.
Insights
Analyzing...
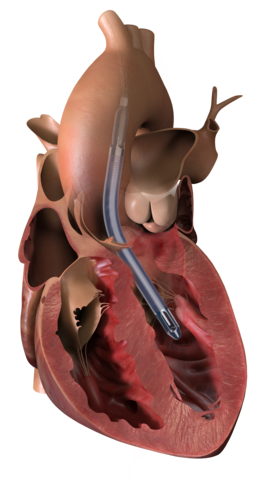
The Impella 5.5 with SmartAssist heart pump delivers full cardiac support, allowing the heart to rest and enabling the heart to achieve its natural pumping function without additional support. This heart pump is designed for long-duration support, enables patient mobility and optimizes recovery by using real-time intelligence. (Graphic: Business Wire)
The analysis examined 143 consecutive patients with cardiogenic shock due to myocarditis who received Impella support or Impella plus VA ECMO support, known as ECpella. These patients are included in the J-PVAD registry, a registry conducted by 10 Japanese professional societies, including the
“These findings further demonstrate the potential of increasing native heart recovery in myocarditis patients through the use of Impella, which is an important consideration given the limited number of heart transplants,” said lead investigator
Myocarditis is the inflammation of the heart muscle often caused by a viral infection. This inflammation may affect the heart’s electrical system and cause the muscle to enlarge, which has the potential to weaken the heart and force it to work harder to circulate blood and oxygen to the rest of the body. Ultimately, this could lead to heart failure.
According to a
“Myocarditis is a growing epidemic in the COVID-19 era. It is exciting to see data from this study demonstrates the potential for Impella support to improve patient outcomes in this very sick patient population,” said
In
In
ABOUT IMPELLA HEART PUMPS
Impella 2.5, Impella CP®, Impella CP with SmartAssist, Impella 5.0®, Impella LD® and Impella 5.5® with SmartAssist® are
Impella Left Ventricular (LV) Support Systems are also authorized for emergency use by HCPs in the hospital setting for providing temporary (≤ 4 days for Impella 2.5, Impella CP, and Impella CP with SmartAssist; and ≤ 14 days for Impella 5.0 and Impella 5.5 with SmartAssist) LV unloading and support to treat critical care patients with confirmed COVID-19 infection who are undergoing ECMO treatment and who develop pulmonary edema while on V-A ECMO support or late cardiac decompensation from myocarditis while on V-V ECMO support. The authorized Impella LV Support Systems have neither been cleared or approved for the authorized indication for use. The Impella RP and Impella LV Support Systems have been authorized for the above emergency use by FDA under an EUA and have been authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
ABOUT
Based in
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in
View source version on businesswire.com: https://www.businesswire.com/news/home/20220920005519/en/
Media Contact:
Associate Director,
+1 (978) 882-8491
jleary@abiomed.com
Investor Contact:
Executive Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com
Source:







