FDA Grants Breakthrough Device Designation to Impella ECP, the World’s Smallest Heart Pump
The United States Food and Drug Administration (FDA) has granted breakthrough device designation to Abiomed’s (NASDAQ: ABMD) Impella ECP expandable percutaneous heart pump. The designation means the FDA will prioritize Impella ECP’s regulatory review processes including design iterations, clinical study protocols and pre-market approval (PMA) application.
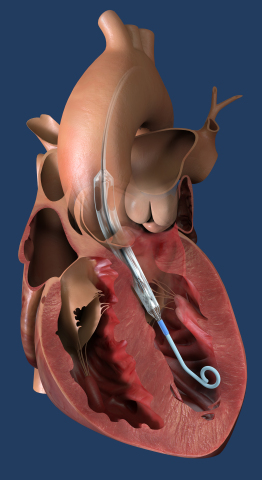
Impella ECP, the world's smallest heart pump, is placed percutaneously into the heart's left ventricle, expands, and supports the heart’s pumping function, providing flow greater than 3.5 L/min. (Photo: Business Wire)
Impella ECP is the smallest heart pump in the world and the first to be compatible with small bore access and closure techniques. It measures 9 French (3 millimeters) in diameter upon insertion and removal from the body. While in the heart, it expands to support the heart’s pumping function, providing flow greater than 3.5 L/min.
The FDA granted breakthrough device designation in part based on positive clinical data from the first 21 Impella ECP patients treated as part of an FDA regulated early feasibility study. In granting the designation, the FDA determined Impella ECP meets the FDA’s stringent requirements for a breakthrough device.
“This is yet another validation from the FDA of the clinical benefits of Impella technology and an affirmation of the innovative nature of Impella ECP which, due to its smaller vascular access size, has the potential to provide even safer procedures and be available to more patients who need hemodynamic support for coronary revascularization,” said Chuck Simonton, MD, Abiomed’s chief medical officer.
In the United States, an estimated 440,000 patients are indicated and yet undertreated for high-risk PCI. Impella ECP’s size may enable more physicians to provide critical hemodynamic support to coronary artery disease patients who need it.
The first Impella ECP patient in the world is Robert Matthews, an 80-year-old retired auto worker from Detroit, a father of four, and grandfather of eleven. For more than 20 years, Robert lived with heart disease and endured multiple procedures. In 2020, he was referred to Amir Kaki, MD, an interventional cardiologist and director of mechanical circulatory support at Ascension St. John Hospital in Detroit. Dr. Kaki discovered multiple blockages and poor heart function and identified Robert as an appropriate candidate for a Protected PCI with Impella. Robert became the first patient in the world treated with Impella ECP when Dr. Kaki inserted the heart pump prior to opening blockages and placing stents.
Two days later, Robert returned home, and his family and friends immediately noticed his renewed energy. Today, Robert is grateful for the cutting-edge technology that restored his quality of life.
Caution: Impella ECP is an investigational device, limited by federal law to investigational use only.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI), such as stenting or balloon angioplasty, to reopen blocked coronary arteries.
The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5® with SmartAssist® are U.S. FDA approved to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support and oxygenation. Our products are designed to enable the heart to rest by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210818005373/en/







