Early Feasibility Study Demonstrates Successful Use of Abiomed’s preCARDIA Technology
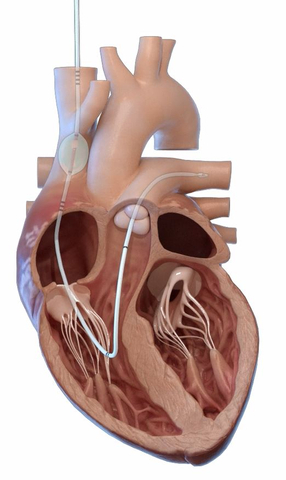
The preCARDIA system is placed in the heart to intermittently occlude the superior vena cava. (Graphic: Business Wire)
Despite available pharmaceutical treatments, heart failure is the leading cause of hospitalization in patients older than 65 years of age. Diuretics may help to improve heart failure symptoms and heart function by reducing fluid overload. However, diuretics take time to work and may be ineffective in patients with chronic heart failure. preCARDIA is designed to reduce filling pressure as blood enters the heart and lungs, which may enable the heart and kidneys to work more effectively, potentially providing therapy for patients non-responsive to diuretics, estimated to be approximately 300,000 of the one million
The multicenter, prospective, single-arm VENUS-HF Early Feasibility Study examined 30 patients with ADHF who were assigned preCARDIA therapy for 12 or 24 hours. The primary endpoint was a composite of major adverse events through 30 days. The study found:
-
Freedom from device- or procedure-related major adverse events in
100% of patients (n=30/30) -
Successful placement, activation and removal of preCARDIA in
97% of patients (n=29/30) -
Right atrial pressure decreased
34% from baseline (p<0.001) -
Pulmonary capillary wedge pressure decreased
27% from baseline (p<0.001) -
Urine output increased by
130% from pretreatment values (p<0.01) -
Net fluid output increased by
156% from pretreatment values (p<0.01)
“The study met the safety and feasibility endpoints and suggests for the first time that with the preCARDIA system it is possible to rapidly reduce cardiac filling pressures and augment urine output by intermittently occluding the superior vena cava in patients with ADHF,” said
The results support additional study of preCARDIA. In November, the
preCARDIA is an investigational device, limited by federal law to investigational use only.
ABOUT
Based in
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in
View source version on businesswire.com: https://www.businesswire.com/news/home/20220112005319/en/
Media:
Director of Communications
+1 (978) 882-8408
tlangford@abiomed.com
Investors:
Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com
Source:







