Abiomed Successfully Completes All Impella Post-Approval Studies for High-Risk PCI, Cardiogenic Shock, Post-Cardiotomy Cardiogenic Shock and Right Heart Failure
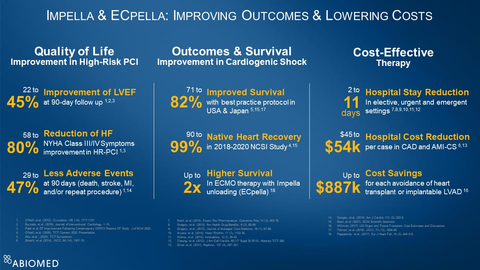
Figure 1
The FDA typically requires post-approval studies for medical devices that receive a PMA, the FDA’s highest level of regulatory approval. FDA post-approval studies use high-quality prospective data to confirm that the clinical study data submitted to the FDA to receive a PMA applies to a broader, real-world population of patients.
In total,
“This significant regulatory milestone once again confirms the safety and efficacy of Impella across a variety of clinical indications. I applaud the physician-researchers who led these studies and thank the patients who participated in them,” said
The totality of Impella data collected in the
-
Impella-supported Protected PCI improves quality of life, with a
22% to45% improvement in left ventricular ejection fraction at 90-day follow up1, 2, 3, a58% to80% reduction in New York Heart Association Class III and IV symptoms1, 3 and29% to47% fewer adverse events at 90 days1, 14. -
Impella improves outcomes in cardiogenic shock, with
71% to82% survival with best practice protocols5, 15, 17,90% to99% native heart recovery in the 2018 to 2020 National Cardiogenic Shock Initiative (NCSI) Study4, 15 and up to two-times higher survival for ECMO therapy when it is combined with Impella unloading (known as ECpella)18. -
Impella is a cost-effective therapy that reduces hospital length-of-stay two to eleven days in elective, urgent and emergent settings7, 8, 9, 10, 11, 12, reduces hospital cost per case by
$45,000 $54,000 $887,000
Impella is the most studied heart pump in the history of the FDA, with studies being conducted from 2006 to the present. Real-world data exists on nearly 200,000 Impella patients (see figures 2 & 3) and Impella is the subject of more than 1,200 peer-reviewed publications. It is included in 13 clinical society guidelines.
The clinical data and best practices learned from all Impella studies performed with the FDA, the
______________________________
- O’Neill, et al. (2012). Circulation, 126 (14), 1717-1727.
-
Burzotta, et al. (2019).
Journal of Interventional Cardiology , 1–10. - Patel et al. EF Improvement Following Contemporary HRPCI: Restore EF Study. J of SCAI 2022.
- O’Neill, et al. (2020). TCT Connect 2020 Presentation.
- Ako, et al. (2022). TCT Symposium.
- Stretch, et al. (2014). JACC, 64 (14), 1407-15.
- Maini, et al. (2014). Expert Rev Pharmacoecon Outcomes Res, 14 (3), 403-16.
- Gregory, et al. (2013). Am Health Drug Benefits, 6 (2), 88-99.
-
Gregory, et al. (2013).
Journal of Managed Care Medicine , 16 (1), 61-69. - Aryana, et al. (2014). Heart Rhythm, 11 (7), 1122-30.
- Wohns, et al. (2014). Innovations, 9 (1), 38-42.
- Cheung, et al. (2012). J Am Coll Cardiol, 60 (17 Suppl B) B110. Abstract TCT-385.
- Silver, et al. (2017). Nephron, 137 (4), 297–301.
- Dangas, et al. (2014). Am J Cardiol, 113 (2), 222-8.
- Basir, et al. (2021). SCAI Scientific Sessions.
- Milliman. (2017). US Organ and Tissue Transplant Cost Estimates and Discussion.
- Tehrani, et al. (2019). JACC, 73 (13), 1659-69.
- Pappalardo, et al. (2017). Eur J Heart Fail, 19 (3), 404-412.
ABOUT IMPELLA HEART PUMPS
Impella CP with SmartAssist® is
Impella CP with SmartAssist® and Impella 5.5® with SmartAssist® are
Impella RP® with SmartAssist is
ABOUT
Based in
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in
View source version on businesswire.com: https://www.businesswire.com/news/home/20221020005716/en/
For further information:
Media:
Associate Director,
+1 (978) 882-8491
jleary@abiomed.com
Investors:
Executive Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com
Source:







