AbCellera-Discovered Antibody, Bebtelovimab, Receives U.S. FDA Emergency Use Authorization for the Treatment of Mild-to-Moderate COVID-19
AbCellera (Nasdaq: ABCL) has received Emergency Use Authorization (EUA) from the U.S. FDA for bebtelovimab (LY-CoV1404), its antibody treatment for mild-to-moderate COVID-19 in high-risk patients. Bebtelovimab effectively neutralizes the Omicron variant, including BA.2, demonstrating broad efficacy against circulating SARS-CoV-2 variants. The authorized dosage is 175 mg as an intravenous injection. Additionally, Lilly has contracted to supply up to 600,000 doses to the U.S. government by March 31, 2022, and an option for 500,000 more by July 31, 2022.
- Emergency Use Authorization received for bebtelovimab to treat high-risk COVID-19 patients.
- Effective against Omicron variant and all known variants of concern.
- Partnership with Lilly for supply agreement of up to 600,000 doses.
- None.
Insights
Analyzing...
- Bebtelovimab (LY-CoV1404) neutralizes Omicron, including the subvariant BA.2, as demonstrated by pseudovirus and/or authentic virus data
- Previously reported data show that bebtelovimab has broad and potent neutralization of all other known circulating SARS-CoV-2 variants of concern
- Bebtelovimab binds to a rarely mutated region of the SARS-CoV-2 spike protein, suggesting the potential to retain effectiveness against emerging variants
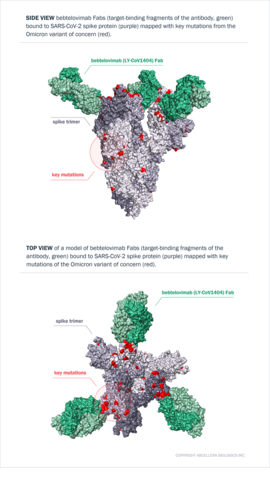
Models of bebtelovimab Fabs bound to SARS-CoV-2 spike protein mapped with key mutations from the Omicron variant of concern. Top: Side view of a model of bebtelovimab Fabs (target-binding fragments of the antibody, green) bound to SARS-CoV-2 spike protein (purple) mapped with key mutations from the Omicron variant of concern (red). Bottom: Top views of a model of bebtelovimab Fabs (target-binding fragments of the antibody, green) bound to SARS-CoV-2 spike protein (purple) mapped with key mutations of the Omicron variant of concern (red). Source: AbCellera
“At the start of the COVID-19 pandemic we and our partners prioritized speed in getting therapies out to patients. This resulted in the discovery of bamlanivimab, the first COVID-19 antibody to reach the clinic and receive FDA Emergency Use Authorization,” said
As previously announced, Lilly entered into a purchase agreement with the
Pseudovirus and authentic virus testing confirm bebtelovimab neutralizes Omicron – currently the predominant variant in the
About AbCellera’s Response to COVID-19
AbCellera initially mobilized its pandemic response platform against COVID-19 in February of 2020, resulting in the discovery of bamlanivimab, the first monoclonal antibody therapy for COVID-19 to reach human testing and to be authorized for emergency use by the
AbCellera’s second monoclonal antibody therapy for COVID-19, bebtelovimab, was developed to combat emerging variants. Pseudovirus and authentic virus testing confirmed bebtelovimab maintains binding and neutralizing activity across currently known and reported variants of concern. It is being studied for the treatment of mild to moderate COVID-19 both as a monotherapy and together with other antibodies.
AbCellera’s efforts to respond to the COVID-19 pandemic have identified thousands of unique anti-SARS-CoV-2 human antibodies. These include bamlanivimab, bebtelovimab, and other antibodies that are in various stages of testing by AbCellera and its partners.
Bamlanivimab and bebtelovimab were developed from antibodies that were discovered using AbCellera’s pandemic response platform, in partnership with the
AbCellera’s pandemic response capabilities were developed over the past four years as part of the
About
AbCellera is a technology company that searches, decodes, and analyzes natural immune systems to find antibodies that its partners can develop into drugs to prevent and treat disease. AbCellera partners with drug developers of all sizes, from large pharmaceutical to small biotechnology companies, empowering them to move quickly, reduce cost, and tackle the toughest problems in drug development. For more information, please visit www.abcellera.com.
AbCellera Forward-looking Statements
This press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. The forward-looking statements are based on management’s beliefs and assumptions and on information currently available to management. All statements contained in this release other than statements of historical fact are forward-looking statements, including statements regarding our ability to develop, commercialize and achieve market acceptance of our current and planned products and services, our research and development efforts, and other matters regarding our business strategies, use of capital, results of operations and financial position, and plans and objectives for future operations.
In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, levels of activity, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors are described under "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and elsewhere in the documents we file with the
Source:
View source version on businesswire.com: https://www.businesswire.com/news/home/20220211005524/en/
Inquiries
Media:
Business Development:
Investor Relations:
Source:







