Pliant Therapeutics Announces Positive Topline Data from a Phase 2a Collagen PET Imaging Clinical Trial of Bexotegrast in Patients with Idiopathic Pulmonary Fibrosis
Pliant Therapeutics announced positive topline data from a 12-week Phase 2a clinical trial of bexotegrast (PLN-74809) in patients with idiopathic pulmonary fibrosis (IPF). The study showed that bexotegrast 160 mg led to a reduction in total lung collagen compared to an increase in the placebo group, suggesting potential reversal of fibrosis. Improvements in forced vital capacity (FVC) and reduced cough severity were observed in bexotegrast-treated patients at all timepoints. The drug was well tolerated with no serious adverse events or discontinuations. The trial involved 10 patients, with 7 receiving bexotegrast and 3 on placebo. PET imaging was used to measure changes in lung collagen levels. The primary endpoint was the change in standardized uptake value (SUV) of a PET ligand that binds to type 1 collagen. Bexotegrast-treated patients showed a reduction in SUV, indicating a decrease in lung collagen. Additionally, improvements in fibrosis biomarkers, FVCpp, and patient-reported cough severity were noted.
- Bexotegrast 160 mg showed a reduction in total lung collagen in IPF patients.
- Improvement in forced vital capacity (FVC) was observed in bexotegrast-treated patients.
- Reduction in cough severity reported in patients treated with bexotegrast.
- Bexotegrast was well tolerated with no serious adverse events over 12 weeks.
- No discontinuations were noted in the bexotegrast treatment group.
- Trial demonstrated potential reversal of fibrosis as indicated by PET imaging.
- Positive result in exploratory endpoints such as FVCpp and fibrosis biomarkers.
- The trial involved only 10 patients, limiting the statistical power of the results.
- Only 3 patients were in the placebo group, which may affect the robustness of the comparative analysis.
- Patients were already on standard of care, primarily nintedanib, potentially influencing the results.
The results from Pliant Therapeutics' Phase 2a trial of bexotegrast in patients with Idiopathic Pulmonary Fibrosis (IPF) show promising developments for this challenging disease. The standout finding is the reduction in total lung collagen in patients treated with bexotegrast, as measured by PET imaging. This suggests a potential reversal of fibrosis, a major breakthrough considering the progressive nature of IPF.
Forced Vital Capacity (FVC) improvement is a critical metric for assessing lung function. Bexotegrast-treated patients showed better FVC across all timepoints compared to placebo, indicating enhanced lung capacity and function. Additionally, the reduction in cough severity reported by patients is significant, as chronic cough in IPF is not only debilitating but also a predictor of disease progression.
The use of PET imaging with a collagen-binding radiotracer is innovative. This method provides more precise and direct insight into the drug's impact on lung fibrosis. The fact that bexotegrast was well-tolerated with no serious adverse events is also a positive outcome, suggesting good patient compliance prospects.
Rating: 1
From a market perspective, the topline results of Pliant Therapeutics' trial are likely to bolster investor confidence. The reduction in total lung collagen and improvements in FVC for IPF patients treated with bexotegrast are compelling. These findings could place Pliant Therapeutics in a stronger position within the competitive landscape of fibrosis treatment, potentially leading to increased market share.
Moreover, the ability to demonstrate efficacy in a short 12-week span is advantageous, as it indicates quicker timelines potentially for regulatory approval and market entry. Investors will note the drug's safety profile, with no serious adverse events, which reduces the risk of regulatory setbacks.
However, it's important to consider the small sample size of the trial. With only 10 patients, the results, while promising, need to be validated in larger, more diverse cohorts to ensure robustness and generalizability.
In the short term, this news is likely to cause a positive reaction in the stock price, but long-term investor sentiment will depend on the outcomes of further trials and eventual regulatory approval.
Rating: 1
Evaluating the financial implications, Pliant Therapeutics' recent clinical trial results have the potential to significantly increase the company's valuation. The positive topline data, including the reduction in total lung collagen and improved FVC, indicate that bexotegrast could potentially fulfill a important unmet medical need in the IPF market. This could attract substantial investment and partnership opportunities.
Successful progression through clinical trials enhances future revenue prospects, given the high cost and market size of IPF therapies. The absence of serious adverse events also suggests fewer hurdles in regulatory approval, which is a critical factor for financial investors.
Nevertheless, the small sample size warrants caution. Investors should look for data from larger Phase 3 trials to validate these findings before making long-term investment decisions. Also, the competition in the fibrosis therapeutic market is growing and Pliant will need to continue to demonstrate superior efficacy and safety to capture significant market share.
Given the current data, this news should positively influence short-term stock performance, with potential for substantial long-term gains if further trials are successful.
Rating: 1
12-week treatment with Bexotegrast 160 mg resulted in reduction of total lung collagen
as measured by PET imaging, compared to an increase on placebo
Improvement in FVC and reduction in cough severity reported in bexotegrast-treated patients
at all timepoints compared to placebo
Bexotegrast 160 mg was well tolerated over 12 weeks of treatment
with no serious adverse events and no discontinuations
SOUTH SAN FRANCISCO, Calif., May 14, 2024 (GLOBE NEWSWIRE) -- Pliant Therapeutics, Inc. (Nasdaq: PLRX), today announced topline data from a 12-week, randomized, double-blind, placebo-controlled trial of bexotegrast (PLN-74809) conducted at Massachusetts General Hospital evaluating change in total collagen levels in the lungs of patients with idiopathic pulmonary fibrosis (IPF). IPF is a disease characterized by excessive collagen deposition in the lung.
Bexotegrast-treated patients showed reduced total lung collagen post treatment as measured by positron emission tomography (PET) imaging, compared to increased total lung collagen in the placebo group, suggesting potential reversal of fibrosis. Bexotegrast-treated patients demonstrated improvements in forced vital capacity (FVC) and reduction in cough severity across all timepoints compared to placebo. Bexotegrast 160 mg was well tolerated over 12 weeks with no drug-related serious adverse events (SAEs) and no discontinuations.
The trial evaluated bexotegrast at a once-daily dose of 160 mg versus placebo, and measured change in total lung collagen in 10 patients with IPF after 12 weeks of treatment. Patients underwent PET imaging with a collagen-binding radiotracer at baseline and Week 12.
The trial enrolled 7 patients in the active arm and 3 in the placebo arm. Eight out of 10 enrolled patients were on standard of care with the majority of those on nintedanib.
Bexotegrast 160 mg-Treated Patients Showed Reduced Total Lung Collagen Levels After 12 Weeks of Treatment, Suggesting Potential Reversal of Fibrosis
The primary endpoint of the trial was an evaluation of the change in standardized uptake value (SUV) of 68GA-CBP8, a PET ligand that binds to type 1 collagen. Type 1 collagen is the predominant collagen type produced in the lungs as a result of IPF.1 An increase in SUV of in the lung indicates increased total lung collagen and potential progression of disease. IPF patients have been shown to exhibit higher SUV values compared to healthy subjects.2 Additionally, patients with increased total lung collagen as measured by PET imaging had an increased risk of death.3
After 12 weeks of treatment, bexotegrast-treated patients showed a reduction in SUV in the lung compared to an increase seen in placebo. This reduction in SUV indicates reduced total lung collagen in the treated group, suggesting potential reversal of fibrosis.
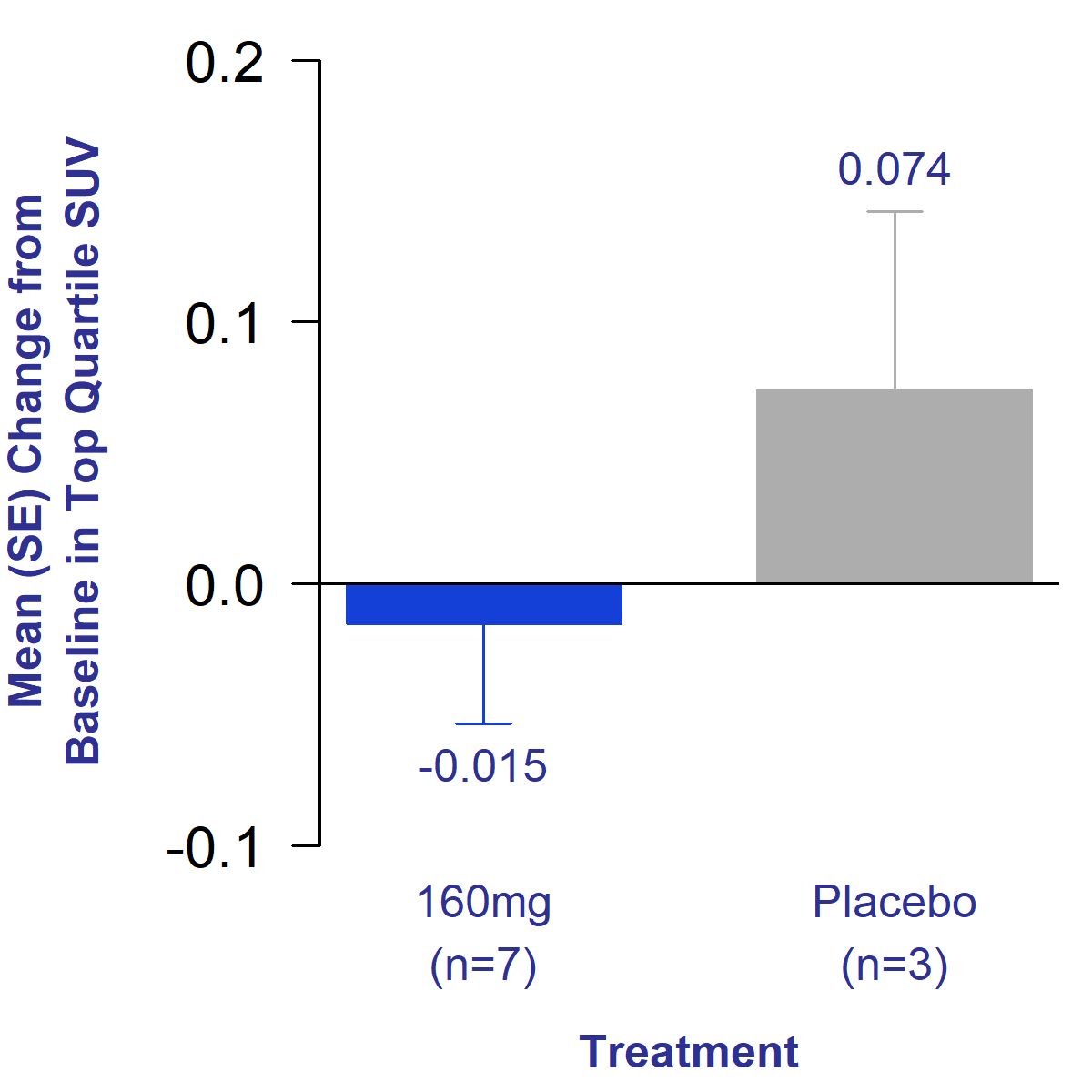
Figure 1. Mean Change from Baseline in Uptake of Collagen PET Tracer After 12 Weeks
Bexotegrast-Treated Patients Demonstrated Improvements in FVC, Cough Severity, and Fibrosis Biomarkers Across All Time Points
The trial's exploratory efficacy endpoints assessed changes in FVC, forced vital capacity percent predicted (FVCpp), patient reported cough severity, and fibrosis biomarkers. Bexotegrast-treated patients experienced improved lung function, as measured by FVC and FVCpp, with a clear separation from placebo across all timepoints.
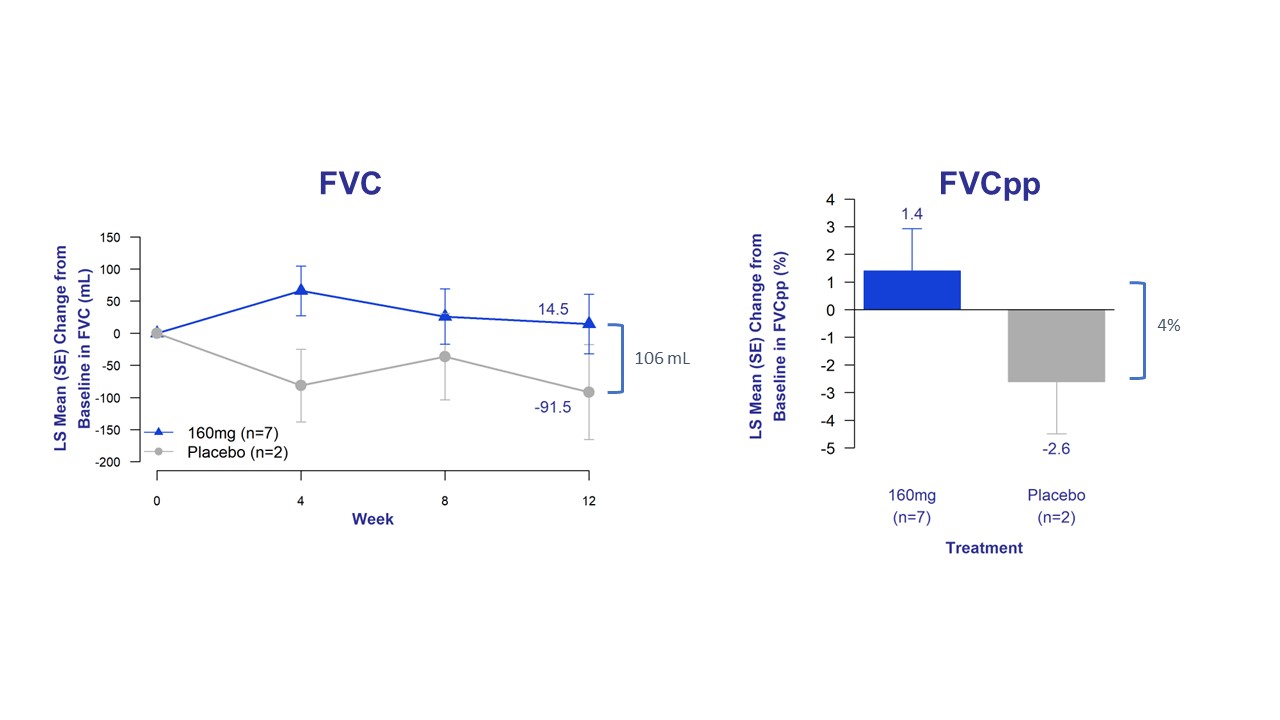
Figure 2. Change in FVC and FVCpp from Baseline of Bexotegrast 160 mg Over 12 Weeks
Chronic cough in IPF is often refractory and debilitating.4 It is an independent predictor of disease progression and may predict time to death or lung transplantation.5 Across all timepoints, bexotegrast-treated patients experienced reduced patient-reported cough severity as measured by the cough visual analog scale (VAS) compared to placebo patients who experienced increased cough severity at all timepoints.
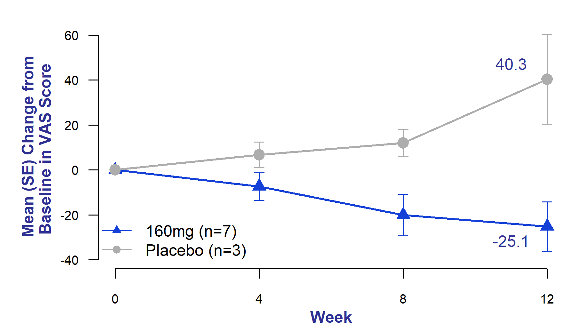
Figure 3. Mean Change from Baseline in Cough Severity Visual Analog Scale (VAS)
of Bexotegrast 160 mg Over 12 Weeks
Elevated integrin beta-6 plasma levels have been associated with interstitial lung disease (ILD) progression as defined by mortality, transplant or ≥
The trial’s secondary endpoint was the evaluation of the safety and tolerability of bexotegrast. Bexotegrast was well tolerated at a dose of 160 mg over 12-weeks of treatment with no serious adverse events (SAE) reported. Most frequently reported treatment-emergent adverse events (TEAEs) were mild in severity with no trial discontinuations.
“These imaging data continue to demonstrate the antifibrotic mechanism of action of bexotegrast and build on previous results, including from our INTEGRIS-IPF Phase 2a trial,” said Éric Lefebvre, M.D., Chief Medical Officer at Pliant Therapeutics.
“Results from this first study using collagen PET imaging to assess a therapeutic intervention highlight the possible utilization of this novel technology to identify potentially disease-modifying antifibrotic IPF therapies in short-term studies,” said Sydney Montesi, M.D., Clinician-Researcher, Division of Pulmonary and Critical Care Medicine at Massachusetts General Hospital and Principal Investigator in the trial.
A slide deck with the topline data from this trial is available under the Investors & Media section of the Pliant website at www.PliantRX.com.
Phase 2a PET Imaging Trial (NCT05621252)
This was a Phase 2a, 12-week, single-center, randomized, double-blinded, placebo-controlled trial that evaluated bexotegrast at a once-daily dose of 160mg or placebo on levels of total collagen deposition in the lungs of participants with IPF. Patients were randomized in a 2:1 ratio of (active:placebo) and stratified based on use of standard of care IPF therapy. Participants underwent positron emission tomography (PET) imaging with a radiotracer, 68Ga-CBP8, that binds to total collagen at Baseline and at Week 12. The trial’s primary endpoint was the assessment of change in Baseline of type 1 collagen in the lung and the secondary endpoint was the evaluation of the safety and tolerability of bexotegrast. The trial's exploratory efficacy endpoints assessed changes in forced vital capacity (FVC) and forced vital capacity percent predicted (FVCpp), changes in patient reported cough severity, and changes in fibrosis biomarkers. Bexotegrast-treated patients demonstrated improvements across all the exploratory efficacy endpoints compared to placebo.
About Pliant Therapeutics, Inc.
Pliant Therapeutics is a late-stage biopharmaceutical company and leader in the discovery and development of novel therapeutics for the treatment of fibrotic diseases. Pliant's lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual selective inhibitor of αvß6 and αvß1 integrins that is in development in the lead indications for the treatment of idiopathic pulmonary fibrosis, or IPF, and primary sclerosing cholangitis, or PSC. Bexotegrast has received Fast Track Designation and Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) in IPF and PSC and Orphan Drug Designation from the European Medicines Agency in IPF and PSC. Pliant has initiated BEACON-IPF, a Phase 2b/3 trial of bexotegrast in IPF. Pl Pliant is conducting a Phase 1 study for its third clinical program, PLN-101095, a small molecule, dual-selective inhibitor of αvß8 and αvß1 integrins, that is being developed for the treatment of solid tumors. Pliant has received regulatory clearance for the conduct of a Phase 1 study of PLN-101325, a monoclonal antibody against integrin α7β1 targeting muscular dystrophies. For additional information, please visit: www.PliantRx.com. Follow us on social media X, LinkedIn, Facebook and YouTube.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. These statements include those regarding the safety, tolerability, pharmacodynamics and therapeutic potential of bexotegrast; our plans for the future development of bexotegrast, PLN-101325 and PLN-101095; bexotegrast’s potential to become a treatment for IPF and the potential future utilization of PET imaging technology to identify IPF therapies. Because such statements deal with future events and are based on our current expectations, they are subject to various risks and uncertainties and actual results, performance or achievements of Pliant Therapeutics could differ materially from those described in or implied by the statements in this press release. These forward-looking statements are subject to risks and uncertainties, including those related to the development and commercialization of our product candidates, including any delays in our ongoing or planned preclinical or clinical trials, the impact of current macroeconomic and marketplace conditions, including the effects of health epidemics and pandemics, such as COVID-19, on our business, operations, clinical supply and plans, our reliance on third parties for critical aspects of our development operations, the risks inherent in the drug development process, the risks regarding the accuracy of our estimates of expenses and timing of development, our capital requirements and the need for additional financing, including the availability of additional term loans under our loan facility, and our ability to obtain and maintain intellectual property protection for our product candidates. These and additional risks are discussed in the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Annual Report on Form 10-K for the period ended December 31, 2023, as updated in our Quarterly Report on Form 10-Q for the period ended March 31, 2024, each available on the SEC's website at www.sec.gov. Unless otherwise noted, Pliant is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
Investor and Media Contact:
Christopher Keenan
Vice President, Investor Relations and Corporate Communications
Pliant Therapeutics, Inc.
1 Kuhn C 3rd, et al. 1989. Am Rev Respir Dis. Dec;140(6):1693-703.
2 Montesi SB, et al. 2019. Am J Respir Crit Care Med 200:258–261.
3 Justet, A. et al. 2017. Respir Res 18, 74.
4 van Manen MJG et al. Eur Respir Rev 2016; 25: 278–286.
5 Ryerson CJ et al. Respirology 2011; 16: 969–975.
6 Organ LA et al. Respir Res. 2019 Jul 12;20(1):148.
7 Bowman WS et al. Lancet Respir Med. 2022 Jun;10(6):593-602.
Figures accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/aa757c2b-a287-4257-9c63-b9f395966616
https://www.globenewswire.com/NewsRoom/AttachmentNg/6b988e76-1c51-4f89-a68d-51e320a285a2
https://www.globenewswire.com/NewsRoom/AttachmentNg/af7a4480-a23e-4062-85ab-930dac489703

FAQ
What were the results of the Phase 2a clinical trial of bexotegrast in IPF patients?
How was the change in lung collagen measured in the bexotegrast trial?
What side effects were observed in the bexotegrast trial?
What was the duration of the bexotegrast Phase 2a clinical trial?







